
Month: January 2011


More Than A Band-Aid For A Broken Heart: New Technology May Regenerate Failing Heart Tissue
For patients who have survived a heart attack, things get worse before they get better if they get better at all. Nearly 2 million people worldwide suffer from permanent heart damage due to lack of an appropriate therapy after a heart attack.

Breakthrough Applications of Apple Technology Deliver a New Approach to HealthCare
On January 25th, 2011, at 8am at the Apple Store on Fifth Ave, Dr. Ali Sadrieh, a Beverly Hills-based surgeon gave an amazing presentation on the effectiveness of the paperless system as he applies it in his office. He lead the discussion into the new ingenious software Mac Practice MD 4.1 and hardward provided by Apple to help the doctors of today run a more effective practice.

A Comparison Between European and U.S. Venture Capital Industries
According to an article in the Independent (UK), 84 percent of participants at a recent biotech conference in Monaco cited “funding” as the biotechnology industry’s biggest challenge.

And The Beat Goes On: Lifesaving Heart Surgery Technology Unveiled
The very life support system that carries a person throughout his or her life, the heart is one of the first things to develop in a fetus.
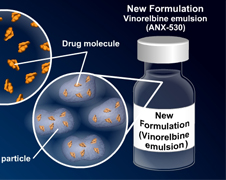
ADVENTRX Pharmaceuticals Takes the Next Step In Approval of ANX-530
ADVENTRX Pharmaceuticals, Inc. (NYSE Amex: ANX), with its leading innovations in anti-cancer drug development has just announced that they have been successful in sending their ANX-530 (Exelbine) New Drug Application (NDA) to the U.S. Food and Drug Administration (FDA) with a Prescription Drug User Fee Act (PDUFA) date of September 1, 2011.

OneMedForum 2011 kicks into high gear on Day 2
Day 2 at the OneMed Forum highlighted a series of exciting presentations & networking opportunities.

OneMedPlace Brings 17 Diverse Workshop Sessions With New Inspirations To All Involved
This year at OneMedForum San Francisco, OneMedPlace offered 17 workshop sessions on a variety of subjects helping investors grow their businesses.

A Candid Look At The 510(k) process for FDA Approval
At this year’s OneMedForum SF, InHealth, the Institute for Health and Technology Studies, partnered with OneMedPlace to produce a panel discussion on improving the 510(k) process for FDA approval.

Day 1 at the OneMedForum 2011
The 4th Annual OneMedForum started on January 11th, 2011 with a large gathering of healthcare financiers and executives filling out the Sir Frances Drake Hotel in San Francisco.