Kinetic Concepts (KCI) has received FDA 510(k) clearance for its LifeCell Tissue Matrix. The matrix is used to treat chronic, trauma, surgical and other acute wounds. The product utilizes the company’s Strattice technology, a sterile reconstructive tissue matrix that supports tissue regeneration.
Strattice is derived from pig skin (porcine dermis, as the company likes to say) and treated with a process to remove cells, significantly reducing the likelihood of rejection. Strattice acts as a scaffold, which is revascularized and repopulated with host cells. Ultimately, the one-time pig skin is converting into functional, living tissue.
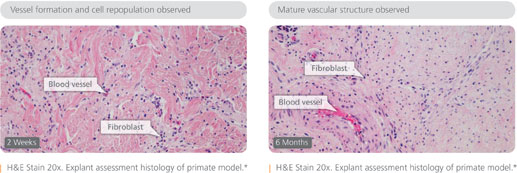
The product can be used to manage of a wide range of wound types, including pressure ulcers, diabetic ulcers and wounds stemming from trauma or surgery. Today’s announcement marks the fourth FDA clearance for Strattice, which is also recommended for soft tissue reinforcement, including hernia repair and breast reconstruction.
The clearance builds upon LifeCell’s significant product portfolio. The company launched in 1994 with AlloDerm, a graft for burn patients. That product has been used in more than one million grafts and implants.
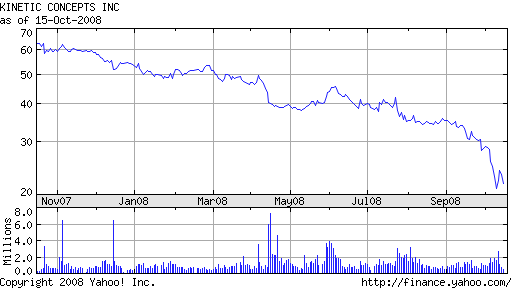
LifeCell was acquired by KCI in April 2008 for $1.7 billion in cash. The company borrowed over $1 billion from Bank of America to finance the deal. The acquisition has done little to inspire investor confidence. With stock trading near $20, down from a high of $65 last October, KCI shareholders have had an abysmal year. Based on its current market capitalization, the company is worth less than what it paid for LifeCell.