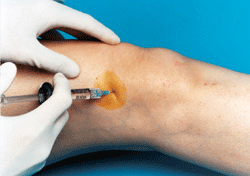
 Anika Therapeutics, a developer of products for tissue protection, healing and repair, has completed patient enrollment for its Monovisc U.S. clinical trial. The randomized, double-blind study is designed to demonstrate that the single-injection viscosupplement therapy safely provides knee pain relief in patients with osteoarthritis.
Anika Therapeutics, a developer of products for tissue protection, healing and repair, has completed patient enrollment for its Monovisc U.S. clinical trial. The randomized, double-blind study is designed to demonstrate that the single-injection viscosupplement therapy safely provides knee pain relief in patients with osteoarthritis.
Monovisc got CE Mark approval last year and is now available in the EU for the treatment of osteoarthritis symptoms in all synovial joints. Commenting on that approval, Charles Sherwood, President and CEO of Anika, said,
“Monovisc is being very well received in Europe, where we launched the product in May. Coincident with that launch, we completed a post-approval study of Monovisc in Europe and the interim results on the product’s performance and duration of action are very strong.”
Clinical data appears to replicate the safety and efficacy of Anika’s popular Orthovisc (a similar treatment for pain resulting from osteoarthritis of the knee) but in a single injection regimen. “Administering this solution through a single injection can offer a convenient option for doctors and patients,” says Sherwood.
The U.S. clinical study for Monovisc was initiated in January 2008; the company expects to begin U.S. commercialization in 2010.
The joint health franchise continues to be a key driver of Anika’s growth. In its most recent quarter, revenue from Orthovisc was up 25 percent year-over-year.
Previously: Monovisc Launch to Build Upon Orthovisc Success