 NeoChord Inc., a venture-backed medical device company focused on minimally-invasive mitral valve repair, this week announced that its mitral valve repair system will be featured in the scientific program at the upcoming 26th Annual Meeting of the European Association for Cardio-Thoracic Surgery (EACTS).
NeoChord Inc., a venture-backed medical device company focused on minimally-invasive mitral valve repair, this week announced that its mitral valve repair system will be featured in the scientific program at the upcoming 26th Annual Meeting of the European Association for Cardio-Thoracic Surgery (EACTS).
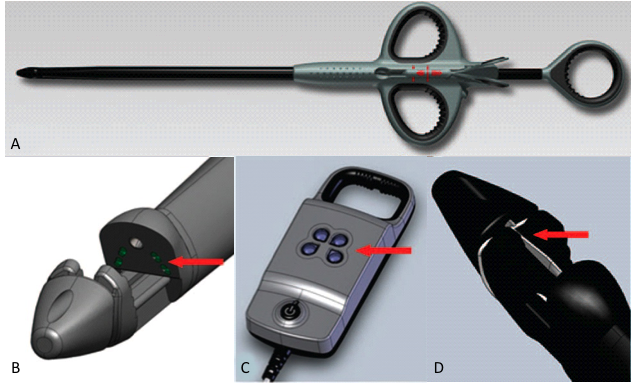
The Minnesota-based company has developed the NeoChord DS1000, a disposable device that replaces damaged chordae by delivering artifical chordae tendinae – neochords – in a beating heart using minimally invasive techniques. The device allows mitral valve repair to be performed on a beating heart through a 2 to 3 inch incision between the ribs in patients with severe, degenerative mitral valve regurgitation.
“In the next three years, I want to see NeoChord’s mitral repair product firmly entrenched in the European surgical community. And, I want to see us well on our way toward commercialization of our mitral repair product in the United States,” said John Seaberg, Chairman and CEO of NeoChord. “I also want to see us leverage our core technology into a platform for other mitral valve procedures, such as edge-to-edge repair, as well as evolve our advanced imaging capabilities.”
Traditional mitral valve repair procedures employ a 3 to 10 inch chest incision – which involves cutting through the sternum, opening the rib cage, stopping the heart and placing the patient on bypass.
This announcement is the next step following the company’s completion of enrollment in its Transapical Artificial Chordae Tendinae [TACT] European clinical trial in September. The trial is a multi-center, nonrandomized, prospective evaluation of NeoChord DS1000, ongoing in Germany, Italy and Lithuania. Interim data from the 30-patient trial shows second-half acute procedure success rates were 94% compatible with early durability results, according to the company. It has also been reported that at 12 and 24-month post-op visits the vast majority of patients operated on using the NeoChord technology experience resolution or reduction of mitral regurgitation up to two years after the procedure.
NeoChord investors include Mayo Clinic, Clarion Health Ventures, Heron Capital, TGap Ventures, Hopen Life Science Ventures, and Cedar Point Capital. The NeoChord technology was developed at the Mayo Clinic, and the company has an exclusive worldwide license to the technology.
Mitral regurgitation is the most common form of heart valve disease, a disorder of the heart in which the mitral valve does not close properly when the heart pumps out blood. Patients with MR experience abnormal leaking of blood from the left ventricle, through the mitral valve. The blood leaks into the left atrium, causing the left ventricle to contract and regurgitate blood back into the left atrium.
The mitral valve is composed of two valve leaflets containing the chordae tendineae – which connect the valve leaflets to the papillary muscles. A dysfunction of the mitral valve apparatus can cause MR; the most common cause is a result of myxomatous degeneration, a stretching out of the leaflets of the valve and the chordae tendineae.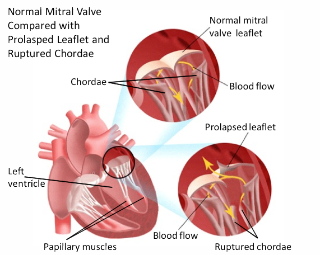
The goal of mitral valve repair is to restore natural leaflet motion and to preserve a large valve opening with leaflets that fit well together, according to the company. NeoChord DS1000 is designed to accomplish this, while also allowing for minimally-invasive surgery. This technique can potentially mitigate the complications associated with open-heart surgery and the stopped heart.
Further, there is immediate confirmation that the regurgitation is reduced, as the heart remains beating.
MR affects approximately 2% of the population, and if left untreated, MR can lead to pulmonary hypertension, arrhythmia and congestive heart failure. It is estimated there is a $2BN market opportunity for mitral valve disease surgical strategies.