Cambridge Heart, of Bedford, MA, has amended its marketing agreement with St. Jude Medical in a way that could significantly expand access to tests for determining candidacy for implantable defibrillators.
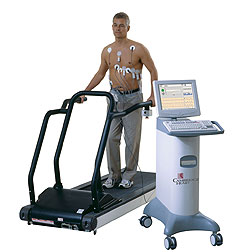 Under the amended terms, St. Jude will be able to market Cambridge Heart’s HearTwave II Microvolt T-Wave Alternans (MTWA) test in North American primary care and internal-medicine markets. The test allows physicians to identify patients who will most likely benefit from implantable cardioverter defibrillators (ICDs). Before, Cambridge Heart was carrying the primary care and internal-medicine load alone, while St. Jude had responsibility for only the cardiology and electrophysiology markets.
Under the amended terms, St. Jude will be able to market Cambridge Heart’s HearTwave II Microvolt T-Wave Alternans (MTWA) test in North American primary care and internal-medicine markets. The test allows physicians to identify patients who will most likely benefit from implantable cardioverter defibrillators (ICDs). Before, Cambridge Heart was carrying the primary care and internal-medicine load alone, while St. Jude had responsibility for only the cardiology and electrophysiology markets.
St. Jude paid $12.5 million in convertible stock at the time the original, three-year agreement was signed, in March; material terms remain the same under the amended deal.
Cambridge Heart will maintain sole responsibility for managing system installation, training new customers and servicing of equipment — a task that could intensify (and become increasingly expensive) with St. Jude’s army making sales in all physician markets.
“Expanding the collaborative partnership provides an opportunity to penetrate the internal medicine and primary care markets more efficiently,” said Cambridge Heart’s Chairman, Robert Khederian, in a statement. ” Our two sales forces have worked very well together over the last few months, and by jointly addressing all markets, we can increase awareness for sudden cardiac death and enhance the adoption of MTWA as an effective tool for identifying high-risk patients.”
An ICD is an electronic device that constantly monitors heart rate and rhythm. When it detects a very fast, abnormal rhythm, it delivers energy to the heart muscle, causing the heart to beat normally. These kind of implants have recently taken a hit, following a number of recalls and a handful of media-hyped related deaths in 2005; sales in 2006 were sluggish. But the Centers for Medicaid and Medicare Services (CMS) shed some light on the issue recently, when it said that only a small proportion of those with ICDs actually received appropriate shocks. The result has been a real need for tests that help determine who’s an appropriate candidate for an implant. To that end, CMS has instituted coverage for Cambridge Heart’s diagnostics. Cambridge estimates that it could prevent ICD placement in as many as 33% of the 1.3 million primary prevention patients. The diagnostic, at 1% the cost of an ICD, could lead to a potential savings of $13 billion.
Whether that could mean a big blow to the companies that make ICDs remains to be seen: “It could eliminate up to a third of the potential market for I.C.D.’s by identifying patients who are unlikely to benefit from the devices,” The New York Times reported last month. The paper goes on to point out, though, that “while the pool would shrink, those left would be more likely to benefit so more doctors might refer them to electrophysiologists, the specialized cardiologists who do the implants.”
Even with the amendment to the St. Jude agreement, Cambridge Heart is sticking to its guidance — $14-$16 million in revenue for 2007 — which may be a sign that the company knows it’s outlined an ambitious goal. It had revenues of just $7.4 in 2006, and a net loss of $10.6 million.