Despite physicians’ best efforts and parents’ reassurance, receiving a shot is a terrifying experience for a young child. And with an ever-increasing population of insulin-dependent diabetics requiring several injections a day, adults also would be pleased to learn of needle-free alternatives.
While needle-free injection technology has been around at least since the 1960s, when Aaron Ismach developed the Jet Injector Gun, to date, the technology has mostly been used by the U.S. military for mass immunizations. Of the 3.2 million American diabetics, most use syringes; in 2006 only .6% of insulin-dependent diabetics in the U.S. used needle-free injectors.
But in the past decade, a number of new devices have emerged that deliver drugs subcutaneously, without the use of sharps. Here are a few of the most promising:
Mini-Ject by Valeritas
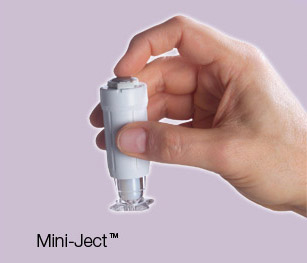 Mini-Ject, made by Parsippany, NJ-based Valeritas, allows patients to self-administer injectable medication. Ease-of-use was the principal consideration in the device’s design and functionality; it’s small and lightweight and can be easily transported in a pocket or purse. Mini-Ject can deliver a wide range of drugs, ranging from small molecules to large proteins, fragile antibodies, and vaccines.
Mini-Ject, made by Parsippany, NJ-based Valeritas, allows patients to self-administer injectable medication. Ease-of-use was the principal consideration in the device’s design and functionality; it’s small and lightweight and can be easily transported in a pocket or purse. Mini-Ject can deliver a wide range of drugs, ranging from small molecules to large proteins, fragile antibodies, and vaccines.
Biojector 2000 by Bioject
 Bioject, of Tualatin, OR, is looking to corner the home-delivery market with its Biojector 2000, which uses a sterile single-use syringe. The plastic syringe is the only part of the system that comes in contact with the patient’s skin. After each injection, the used syringe is discarded and a new one is inserted for the next injection. Because the syringe is needle-free, it does not have to be discarded in a “sharps” container, which decreases the amount of costly, hazardous waste. The disposable component provides a steady source of revenue for Bioject.
Bioject, of Tualatin, OR, is looking to corner the home-delivery market with its Biojector 2000, which uses a sterile single-use syringe. The plastic syringe is the only part of the system that comes in contact with the patient’s skin. After each injection, the used syringe is discarded and a new one is inserted for the next injection. Because the syringe is needle-free, it does not have to be discarded in a “sharps” container, which decreases the amount of costly, hazardous waste. The disposable component provides a steady source of revenue for Bioject.
Bioject’s systems are not painless. Some medications and vaccines can cause burning and stinging sensations because of their formulations, which are independent of the injection method. For highly needle-phobic children, the Biojector 2000 can attach “Elephant Ears,” to help reduce anxiety.
PenJet by PenJet Corporation
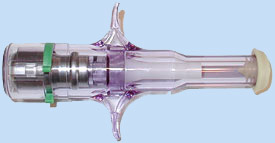 PenJet, the signature product of Santa Monica, CA-based PenJet Corporation, is another small, inexpensive, disposable jet injector that delivers a single dose of medication. It is powered by self-contained, compressed inert gas and can be shipped pre-filled with a drug or filled prior to injection.
PenJet, the signature product of Santa Monica, CA-based PenJet Corporation, is another small, inexpensive, disposable jet injector that delivers a single dose of medication. It is powered by self-contained, compressed inert gas and can be shipped pre-filled with a drug or filled prior to injection.
Ease-of-operation and low cost make PenJet applicable to everything from mass inoculations to home use. The device is available for needle-less subcutaneous, intradermal and some intramuscular injections; dosage size can be as large as 0.5 ml or as small as 0.1 ml.
PassPort by Altea Therapeutics
 Tucker, GA-based Altea Therapeutics took a different route when designing its PassPort System. Forgoing the jet, Altea created a device with a single-use disposable patch and a re-useable handheld applicator. The transdermal patch is attached to an array of metallic filaments known as the porator. Pressing the activation button of the applicator releases a single pulse of electrical energy to the porator, where it is converted into thermal energy. The rapid conduction of this thermal energy into the surface of the skin painlessly ablates the outer layer of the skin, creating a series of microchannels that allow the drug to enter the body.
Tucker, GA-based Altea Therapeutics took a different route when designing its PassPort System. Forgoing the jet, Altea created a device with a single-use disposable patch and a re-useable handheld applicator. The transdermal patch is attached to an array of metallic filaments known as the porator. Pressing the activation button of the applicator releases a single pulse of electrical energy to the porator, where it is converted into thermal energy. The rapid conduction of this thermal energy into the surface of the skin painlessly ablates the outer layer of the skin, creating a series of microchannels that allow the drug to enter the body.
The device can deliver a variety of drugs via the skin, including proteins, peptides, carbohydrates, and water-soluble and lipid-soluble small molecules, as well as genes; it also can deliver vaccines.
The PassPort System avoids complications associated with inhaled medications and with the first-pass gastrointestinal and liver metabolism that often occurs after oral administration. The device has the added benefit of providing dosing information and preventing abuse, via a programmable monitoring and lock-out feature.
SonoPrep by Sontra
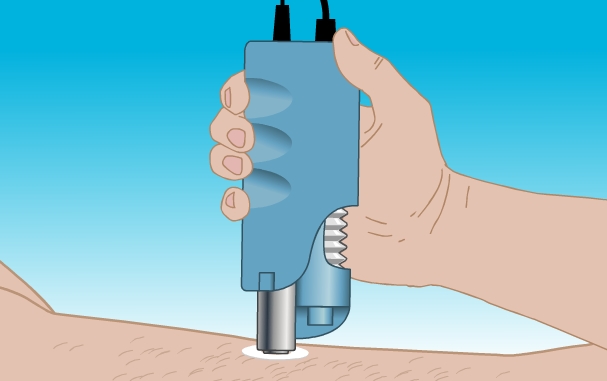 The SonoPrep System, made by Franklin, MA-based Sontra, is another departure from the jet-based injector. SonoPrep uses low-level ultrasound to create a permeable layer on the outermost layer of the skin.
The SonoPrep System, made by Franklin, MA-based Sontra, is another departure from the jet-based injector. SonoPrep uses low-level ultrasound to create a permeable layer on the outermost layer of the skin.
A key feature of the SonoPrep Skin Permeation System is real-time skin conductance feedback. SonoPrep measures the increase in skin conductance (or decrease in skin impedance) during the application of ultrasound and stops the sonocation procedure when the desired level of conductance is achieved.
SonoPrep is currently cleared for the temporary disruption of the outer layer of skin prior to the application of Lidocaine Cream, or for the delivery of dermal anesthesia prior to a needle insertion or IV procedure.