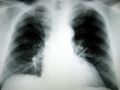
 NeoPharm, a biopharmaceutical company specializing in cancer treatments, has filed an investigational new drug application with the U.S. Food and Drug Administration (FDA) for the treatment of a deadly lung disease. The drug candidate IL13-PE has the potential to be the first effective therapy for idiopathic pulmonary fibrosis (IPF), which kills an estimated 40,000 people in the United States each year.
NeoPharm, a biopharmaceutical company specializing in cancer treatments, has filed an investigational new drug application with the U.S. Food and Drug Administration (FDA) for the treatment of a deadly lung disease. The drug candidate IL13-PE has the potential to be the first effective therapy for idiopathic pulmonary fibrosis (IPF), which kills an estimated 40,000 people in the United States each year.
Illinois-based NeoPharm plans to begin a Phase I clinical trial of IL13-PE in patients with IPF, a progressive disease in which the patient’s lung tissue becomes replaced with scar tissue. Over time, patients experience shortness of breath and a dry, hacking cough. The cause of IPF is unknown, and there is no cure or proven effective treatment for the disease.
NeoPharm’s Phase I study is expected to take place at six to eight sites around the country, with IL13-PE administered to 32 patients in an aerosol form. Researchers plan to assess the safety and efficacy of the drug, as well as the maximum tolerated dose, in patients with advanced IPF. NeoPharm has previously performed animal studies and ex-vivo human tissue studies of IL13-PE.
Other companies working on treatments for pulmonary fibrosis include Arresto Biosciences and mondoBIOTECH. CoTherix is developing a treatment for secondary pulmonary hypertension, a potentially fatal lung condition that can result from IPF.