More partnering news, this time from CombiMatrix and Clarient, who will market and sell a genomics-based cancer test called HemeScan.
HemeScan was developed by CombiMatrix; the test forecasts the probable outcome of chronic lymphocytic leukemia (CLL) and other cancers at the time of diagnosis.
CombiMatrix believes HemeScan could have a “transformative impact” on the clinical stratification of CLL, enabling prognosis at diagnosis and patient-appropriate risk-adapted treatment. HemeScan assays all of the genomic loci for copy number aberrations and incorporates recently-identified genomic markers. The test has been validated by a number of high-profile academic centers.
“The HemeScan test has proven to be a valuable and reliable clinical tool for the management of hematological malignancies (leukemia and lymphomas) and solid tumors characterized by unbalanced chromosomal rearrangements,” said Dr. Shelly Gunn, Medical Director of Combimatrix Molecular Diagnostics. “Along with CLL, the HemeScan test has also been clinically validated for acute lymphoblastic leukemia and myelodysplastic syndrome and is currently being tested for multiple myeloma.”
HemeScan will be marketed by Clarient to pathologists, oncologists, and patients. Based on its existing diagnostic service relationships and a nationwide sales force, Clarient will be well-positioned to sell the test.
Clarient CEO Ron Andrews expects that HemeScan will help drive the company’s hematopathology business. “Our leukemia and lymphoma business line is growing nicely as Clarient continues to adopt innovative technologies and tools to assist local pathologists and oncologist as they manage this disease. We look forward to working with CombiMatrix to support Clarient’s growing lymphoma/leukemia business and as part of our continued focus on personalized medicine.”
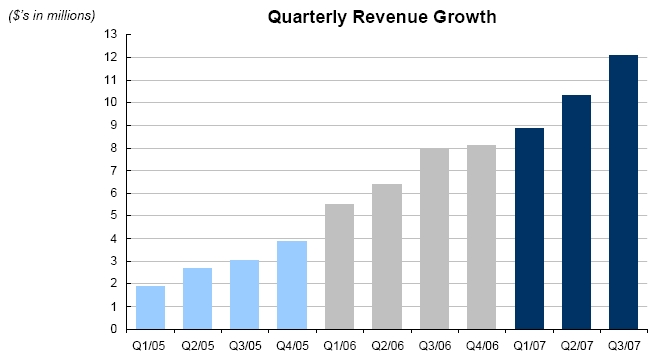
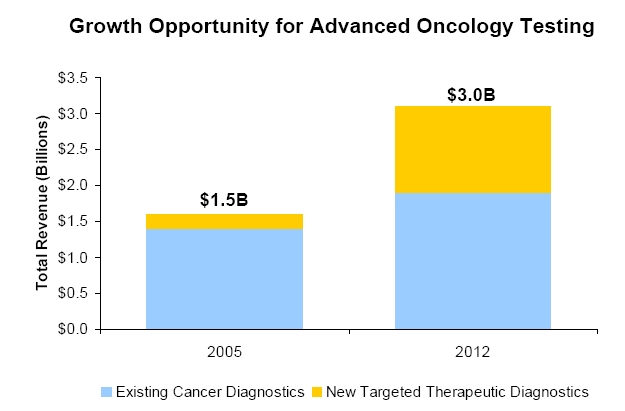
Clarient has experienced 13 sequential quarters of revenue growth. The company believes molecular cancer assessment and personalized therapies will be key drivers in the future oncology market.
Sources: SG Cowen Equity Research, BioBridge Consulting
Chronic lymphocytic leukemia is the most common form of the B-cell lineage lymphoproliferative disorders, accounting for about 30% of all cases in the U.S. and Europe. In the U.S. alone, there are more than 10,000 new cases annually and over 150,000 patients living with the disease. CLL is a clinically heterogeneous disorder with defined risk groups associated with a full spectrum of patient prognostic outcomes.
“The HemeScan test’s technology represents a more precise platform adding greater granularity to assist local pathologists and oncologists in the molecular assessment of this complex disease,” said Ken Bloom, M.D., Clarient’s Chief Medical Officer. “The traditional cytogenetic test used currently does not reliably give us the information we need to take advantage of the therapeutic capabilities available today. Transitioning the industry to more sophisticated technologies undoubtedly will be a major step in providing personalized therapy for individual patients.”