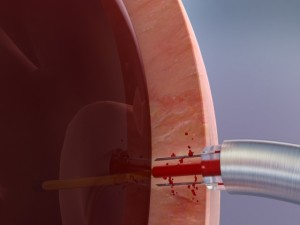
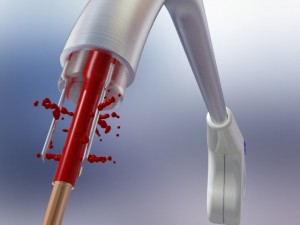
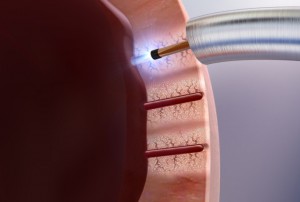
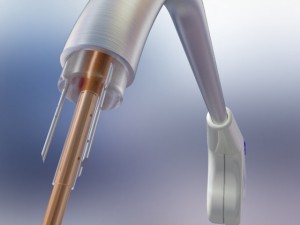
Cardiogenesis, the clinical and market leader in transmyocardial revascularization (TMR), revealed this week that it has enrolled its first two patients for its feasibility study involving the cutting-edge PHOENIX System, a combination delivery system created to treat severe angina (heart pain) using both TMR and the intramyocardial delivery of stem cells simultaneously. This PHOENIX combination delivery system is CE Mark approved and the results of the feasibility study will be used in support of an IDE application to the FDA.
Individuals afflicted with coronary artery disease are traditionally treated by medical therapy; interventional procedures such as angioplasty or stenting; while others receive coronary bypass surgery. Yet coronary artery disease is progressive and it is estimated there are 100,000-200,000 patients are diagnosed each year that are not candidates for intervention or coronary bypass. TMR may be an option for these patients.
On October 7th and 8th of this year, Dr. Guillermo Reyes performed the procedures utilizing the new PHOENIX System technology in Madrid, Spain, during an investigator meeting. “Survival rates have significantly improved for persons with coronary artery disease; however as this population lives longer they can outlive the effectiveness of their prior therapy,” stated Dr. Reyes. “Clinicians are faced with an expanding population of symptomatic patients with no option for conventional treatments. Stem cell therapy with the Cardiogenesis technology has great promise as a solution for this growing clinical problem,” Dr. Reyes then added.
In a TMR procedure, a doctor uses a holmium YAG laser to create channels into the wall of the left ventricle through a tiny incision in the left of the chest. While TMR has been proven to reduce angina amongst the majority of coronary artery disease patients, researchers hope that the additional injection of autologous stem cells may provide even greater angina minimization, as well as improved cardiac functioning amongst the inoperable patients.
|
|
“This is a significant milestone,” commented Cardiogenesis Executive Chairman Paul McCormick. “We will provide the early results of this feasibility study along with the pre-clinical testing being conducted at the Stem Cell Center of the Texas Heart Institute, to support our request to begin a multi-center IDE pivotal trial in the U.S. for the PHOENIX System.” Mr. McCormick believes the PHOENIX System will provide a proprietary growth opportunity for Cardiogenesis, as well as providing effective therapy for inoperable patients with coronary artery disease.
Preeminent researchers planning to participate in this study come from five medical centers across Europe and Asia, including France, India, Italy, Russia, and Spain. Cardiogenesis plans to commence clinical testing at these centers in the upcoming weeks.
Cardiogenesis will be presenting at the OneMedForum SF, January 11th – 13th, 2011.