Regular Sentinel readers may recall previous posts in which I praise Frederick, MD-based BioElectronics Corp. (BIEL), maker of ActiPatch.
The company has experienced strong international growth over the past several months. In an announcement released last week, BIEL noted that during the month of April, the company produced more revenue than in its entire first quarter (Q1 sales were approximately $120,000).
BIEL is expecting sales in May to outpace those of April and June to outpace May. Based on its current and expected order backlogs, it appears the company will continue to ramp sales each month moving forward through 2008.
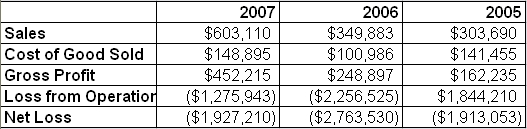
Revenue growth comes at a time when the company has streamlined operations. In 2007 the company moved the bulk of its manufacturing to China, which has significantly reduced costs; BIEL is able to produce ActiPatch at a 70%+ gross margin.
ActiPatch delivers pulsed electromagnetic frequency (PEMF) to accelerate healing of soft tissue injuries. PEMF has been shown to reduce pain and swelling by lessening the body’s inflammatory response. ActiPatch is FDA cleared for the treatment of edema following blepharoplasty (eyelid surgery).
BIEL announced today that the company will initiate studies to investigate the use of ActiPatch in post-operative management of breast augmentation and cesarean section procedures.
According to Andy Whelan, President and CEO of BIEL,
“The use of [ActiPatch] for post-breast augmentation care is already well established and continues to grow at a rapid pace…We are also very excited to be starting the trial for post- operative care for cesarean section. According to the National Center for Health Statistics, approximately one third of all babies in the United States are delivered by cesarean section up from less than approximately 20% just a decade ago. We believe this growing market represents a substantial market opportunity for our company.”
If the results are significant (the device has been clinically proven to reduce swelling and bruising up to 50% in blepharoplasty patients) the studies will contribute clinical data necessary for wider physician acceptance.
One of the company’s stated long-term goals is to receive full approval from the FDA to allow its products to be sold over the counter within the U.S.