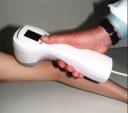
 Electro-Optical Sciences (EOS), developer of The MelaFind System, a hand held imaging device that detects melanomas at an early stage, has completed patient accrual in its blinded pivotal trial.
Electro-Optical Sciences (EOS), developer of The MelaFind System, a hand held imaging device that detects melanomas at an early stage, has completed patient accrual in its blinded pivotal trial.
The trial will serve as the basis for the company’s Pre-Market Approval (PMA) application with the FDA. Conducted at seven centers in the U.S., EOS has enrolled some 1,300 patients and will evaluate over 1,800 suspicious lesions. All suspicious lesions are biopsied and sent for dermatohistopathologic analysis.
In a previous blinded trial examining 352 suspicious pigmented skin lesions, MelaFind had 100% sensitivity in finding a melanoma and achieved 48.4% specificity (compared with the study dermatologists’ sensitivity of 96.4% and specificity of 28.4%). Specificity, in this case, is the probability that an individual who does not have melanoma will be correctly identified as negative; high specificity reduces the need for biopsies.
With assistance provided by MelaFind, physicians could diagnose more melanomas at the earliest curable stage, reducing both treatment costs and the number of unnecessary biopsies. Joseph Gulfo, EOS President and CEO, describes MelaFind as “better, cheaper medicine”.
Results of the pivotal trial are anticipated in the fourth quarter, with PMA filing to follow.
Previously: [Video] Interview with Joseph Gulfo, President and CEO of Electro-Optical Sciences