 Seth Berkley, head of the IAVI or International AIDS Vaccine Initiative (a not-for-profit developing preventive HIV vaccines) believes the majority of HIV vaccines now under development need to be abandoned. These vaccines tweak cell-based immunity, a strategy first explored by Merck.
Seth Berkley, head of the IAVI or International AIDS Vaccine Initiative (a not-for-profit developing preventive HIV vaccines) believes the majority of HIV vaccines now under development need to be abandoned. These vaccines tweak cell-based immunity, a strategy first explored by Merck.
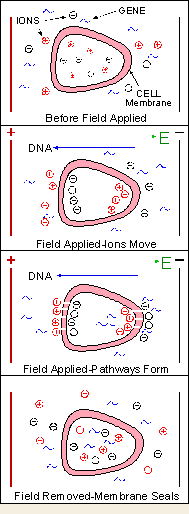
Berkley wants to focus IAVI’s substantial energies on vaccines that mobilize antibodies to neutralize HIV in the bloodstream. Along these lines, IAVI has begun to explore intramuscular delivery of DNA vaccines using Inovio Biomedical’s electroporation technology.
Inovio’s electroporation technology uses controlled electrical pulses to increase cellular uptake of biopharmaceuticals. Intramuscular delivery of DNA vaccines using electroporation technology has been shown in primate studies to boost the immune response by orders of magnitude over DNA plasmid alone. Plasmid-based vaccines induced higher levels of antibodies and T-cell responses when delivered via electroporation, suggesting the potential to provide better protection from infectious diseases such as HIV and hepatitis C.
“The safe and effective delivery of DNA vaccines has proved challenging to date. Electroporation offers a potentially promising solution to this stumbling block,” said Timothy Zamb, head of IAVI’s AIDS Vaccine Development Laboratory.
Inovio and IAVI signed an initial agreement in March 2007. The parties have now extended this agreement by three years. As part of this new agreement, Inovio will provide IAVI with additional electroporation devices to conduct pre-clinical research on HIV genes and vaccine candidates.
Previously: Inovio Biomedical and VGX Pharmaceuticals to Merge