 MiCardia Corporation, developer of treatments for late-stage congestive heart failure and mitral heart valve disease, announced 510(k) clearance this week for its Dynaplasty Annuloplasty Band and Ring. These are the first of MiCardia’s Dynaplasty Technology products to pass FDA muster.
MiCardia Corporation, developer of treatments for late-stage congestive heart failure and mitral heart valve disease, announced 510(k) clearance this week for its Dynaplasty Annuloplasty Band and Ring. These are the first of MiCardia’s Dynaplasty Technology products to pass FDA muster.
Dynaplasty devices can be implanted into the heart and then dynamically adjusted either during a procedure or post-operatively.
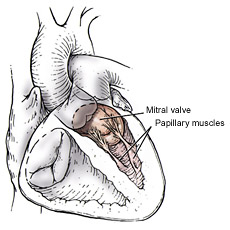
In order to repair the incompetent valve, annuloplasty rings are commonly used in mitral valve repair operations. The rings help maintain the natural shape, motion and flexibility of the annulus. Incomplete or inaccurate repair, or change in the anatomical conditions of the annulus, may result in post operative residual or recurrent regurgitation, requiring high risk repeat surgical intervention and often valve replacement with significant morbidity and mortality.
MiCardia is unique in developing intra-operative, percutaneous and completely non-invasive dynamically adjustable implantable devices.