 According to the American Society for Bariatric Surgery, doctors perform 140,000 gastric bypass surgeries in the United States each year. The most common types are Roux-en-Y gastric bypass and Laparoscopic Adjustable Gastric Banding.
According to the American Society for Bariatric Surgery, doctors perform 140,000 gastric bypass surgeries in the United States each year. The most common types are Roux-en-Y gastric bypass and Laparoscopic Adjustable Gastric Banding.
About 1% of patients who are candidates for gastric bypass surgery actually have the procedure. Mortality risk is the major deterrent to broader use of the Roux-en-Y; although the estimated 1% rate is far lower than the perceived 10% mortality reported by patients and some physicians.
While all surgeries carry some risks, certain factors put patients in a high risk category for gastric bypass. According to a Duke University study these include: A body mass index of greater than 50, male gender, old age, hypertension and pulmonary embolus risk.
GI Dynamics, a Watertown, MA based company is developing EndoBarrier, a technology designed to modify metabolic pathways by lining a portion of the gastrointestinal tract. This is done via an endoscopic procedure. The company’s a non-surgical, noninvasive approach makes it unique among surgical obesity interventions; it offers a potentially safer alternative to gastric bypass with more rapid recovery and lower costs, applicable to wider range of patients.
To date, 118 patients have been outfitted with the liner, in trials conducted in Chile, the Netherlands and the U.S.
Early results from one of those trials, involving 18 type 2 diabetics (12 of whom received the liner and six of whom received a sham endoscopy) was presented last week at the First World Congress on Interventional Therapies for Type 2 Diabetes in New York City. The device appears very promising.
Patients lost an average of 27.5 pounds at the 30-week point. After tracking patients for an average of 31 weeks post-implant, average blood glucose levels fell three to four times more steeply among Endobarrier patients. A previous analysis of the same patient group indicated that Endobarrier improved blood sugar control rapidly within a little as one week.
The study’s authors noted that improvements in blood sugar control appeared to be independent of weight loss indicating that the device could have an impact on diabetics, regardless of weight.
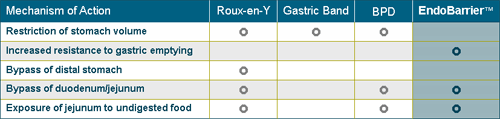
EndoBarrier changes the metabolic pathway by controlling how food moves through the digestive system. The device affects the patient’s uptake of nutrients and calories. As in Roux-en-Y gastric bypass, food bypasses the duodenum and proximal jejunum.
The reversible procedure eliminates hospital stays while minimizing the potential for morbidity and mortality of traditional bariatric surgeries.
“I’m optimistic,” commented Dr. Lee Kaplan, the study’s lead author, “This is one of the more promising devices. But we’re at least a few years away from FDA approval, and this is just an early development phase.”