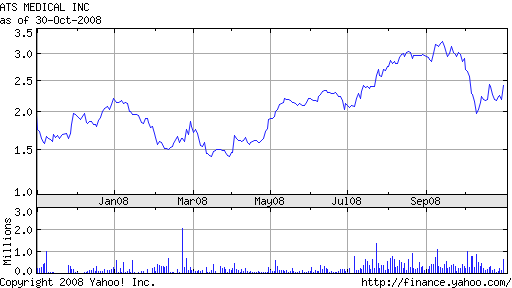
 The FDA has approved ATS Medical’s 3f Aortic Bioprosthesis (3fAB), a poorly named, but potentially revolutionary pericardial aortic tissue valve.
The FDA has approved ATS Medical’s 3f Aortic Bioprosthesis (3fAB), a poorly named, but potentially revolutionary pericardial aortic tissue valve.
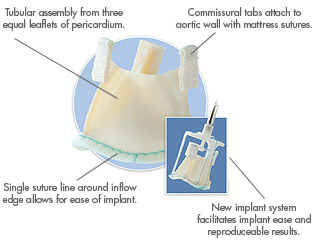
3fAB’s blood flow dynamics and improved stress distribution mimic the functional characteristics of the native valve, offering patients a more durable aortic valve replacement. Six years of clinical experience confirm safety and efficacy.
“The ease of implantation and potential for longer term durability makes this an excellent choice for my patients,” said Dr. Richard Shemin, Chief of Cardiothoracic Surgery at UCLA’s Med School.
Two years ago, ATS acquired 3F Therapeutics, a privately held developer of tissue heart valve products for $58 million. The acquisition brought less-invasive surgical technology to ATS and opened the door to the $400 million U.S. tissue valve market.
A second generation valve, the 3f Enable Aortic Bioprosthesis (Enable) is currently under clinical investigation; ATS submitted an IDE in March 2007.
The Enable valve is the 3f Aortic Bioprosthesis surrounded by a nitinol stent. Nitinol is a memory metal that can be collapsed into a smaller size and shape allowing for easier deployment or implantation (watch the video).
Enable could be a blockbuster. The device has two distinct advantages not presently available to patients who require a tissue valve. Because the Enable valve is essentially sutureless, the surgeon can dramatically reduce procedure time – with conventional valve replacement, placement of sutures can take 20 to 40 minutes. Reduced procedure time increases patient safety and allows more time for concomitant procedures such as coronary artery bypass.
Secondly, because the Enable valve can be packaged into a smaller size than conventional prostheses, the potential for less invasive or keyhole type surgery becomes more practical. Currently, less invasive cardiac surgery is seldom practiced because the large size of the prosthesis requires space for introduction.
ATS expects CE Mark approval for Enable sometime in the first half of 2009.