Irvington-NY based Electro-Optical Sciences (EOS), developer of The MelaFind System, a handheld imaging device that detects melanomas at an early stage, announced last week that its pivotal trial sites have closed: all follow-up data has been received and is now undergoing third-party statistical validation. The company will announce top-line results in the next few weeks.
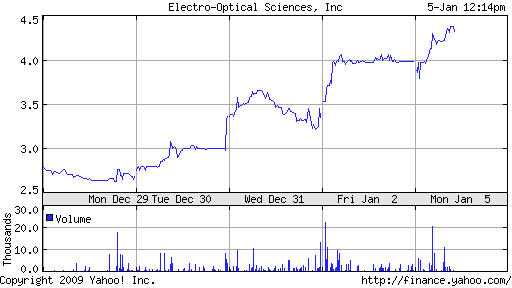
For many investors, the news has been a long time coming. Shares of the company, which had been trading near a 52-week low, have increased by 50 percent.
In a previous blinded trial examining 352 suspicious pigmented skin lesions, MelaFind had 100% sensitivity in finding a melanoma and achieved 48.4% specificity (compared with the study dermatologists’ sensitivity of 96.4% and specificity of 28.4%). Specificity, in this case, is the probability that an individual who does not have melanoma will be correctly identified as negative; high specificity reduces the need for biopsies.
By reducing the number of unnecessary biopsies, Melafind could reduce treatment costs. Joseph Gulfo, EOS President and CEO, describes MelaFind as “better, cheaper medicine”.
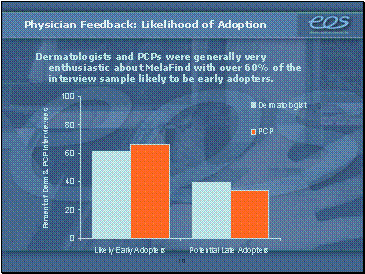
Based on the company’s market research, physicians will seek the MelaFind’s guidance. In a survey of 18 dermatologists and 12 primary care doctors, 60% of both groups indicated that they will be likely early adopters of MelaFind technology. On average, the dermatologists surveyed performed skin checks for melanoma 325 times each month.
Skin cancer is the most common form of cancer in the U.S., with over one million cases diagnosed each year. It also is one of the fastest growing cancers in the U.S. and the leading cause of cancer death in women ages 25-30. Melanoma accounts for only 4% of skin cancer cases, but is responsible for 75% of skin cancer deaths.
Previously: [Video] Interview with Joseph Gulfo, President and CEO of Electro-Optical Sciences