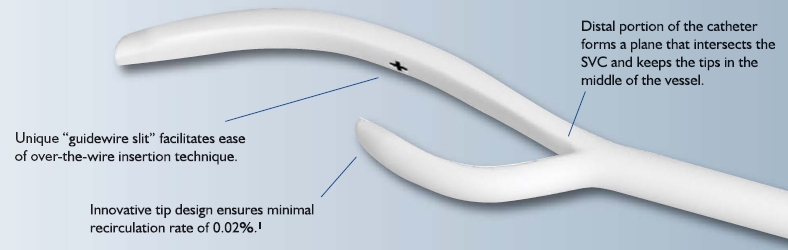
AngioDynamics has initiated a voluntary recall of its Centros central venous catheter for dialysis. The company became aware that the catheter cuff (the component used to anchor the catheter beneath the skin) was inadequately attached to the catheter in some instances, allowing for movement of the catheter within the insertion site, leakage around the site, or the retention of the cuff in the tissue when the catheter was removed.
AngioDynamics belives an outside manufacturer’s production process is to blame.
Since January, the company has shipped approximately 1,500 potentially defective catheters; less than 1% of these have been found problematic. No adverse patient outcomes have been reported.
Pending an FDA review, Centros shipments are expected to resume sometime after December 2008. AngioDynamics expects the total costs associated with the recall to be minimal.