Pittsford, NY-based Biophan Technologies, will move forward with the next phase of the Myotech Circulatory Support System (CSS) engineering and regulatory development program.
The company’s board released $2 million to support efforts which will bring the product closer to commercialization. According to the company, Myotech CSS could be on the market within 24 to 30 months.
The Myotech CSS addresses a significant unmet need – acute circulatory support for patients suffering from sudden cardiac arrest. Today in the U.S., over 260,000 patients suffer in-hospital cardiac arrest every year, and about 80% of those patients do not survive.
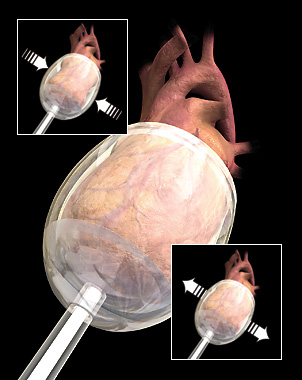
The Myotech CSS consists of an external console and flexible polymer cup that slips over the heart and is designed to restore normal blood flow. It can be deployed quickly for use in acute resuscitation and low cardiac output patient conditions. Myotech CSS, unlike other circulatory support technologies, has no contact with circulating blood, and is expected to significantly reduce the risk of patient complications, such as clotting and stroke, bleeding, and infection.
Biophan’s focus on acute heart failure follows the August sale of its MRI-safety patents to Medtronic for $11 million. In accordance with that deal, Biophan turned over to Medtronic a portfolio of devices including pacemakers, defibrillators and neurostimulators that, unlike many implantable devices on the market, are compatible with MRI.
In October, Biophan increased its stake in Myotech from 44% to 68% following a $1.2 million investment. Biophan believes the device has the potential to generate $100 million in revenues.