 More good news from Endologix, the Irvine, CA-based company mulling over a $98 million buyout bid from hedge fund Elliott Associates.
More good news from Endologix, the Irvine, CA-based company mulling over a $98 million buyout bid from hedge fund Elliott Associates.
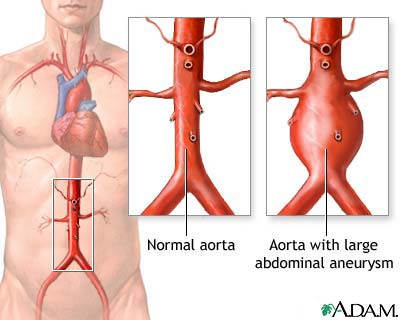
This week, the company’s IntuiTrak Delivery System was approved by the FDA. The System will be used to deliver and deploy the company’s stent graft, for the endovascular repair of abdominal aortic aneurysms (AAA). Endologix plans to conduct a limited market release over the next several months, followed by a full commercial U.S. launch in Q2 2009.
IntuiTrak simplifies delivery of the Powerlink device. The low-profile delivery system is more flexible and smoother, thanks to a hydrophilic coating. The new catheter also offers an integrated sheath to facilitate the introduction of ancillary devices during the endovascular AAA procedure.
That news follows last week’s FDA approval of Powerlink XL, which broadened the company’s treatment indications to include AAA patients with proximal aortic necks between 23 millimeters and 32 millimeters.
Endologix has managed to grow its revenues even as other companies have entered the AAA treatment space: Cook Medical offers the Zenith Fenestrated AAA Endovascular Graft (not FDA approved) and device giant Medtronic has developed the Endurant Abdominal Stent Graft System, FDA approved in April 2008.
Previously: Long Term Data Supports Use of Powerlink