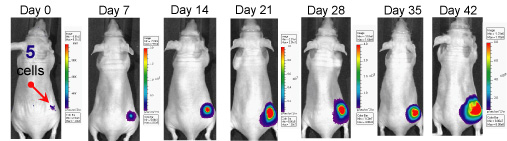
Five cancer cells were injected subcutaneously into the dorsal region near the thigh of mouse. Tumor growth is monitored weekly by an IVIS Spectrum. The tumor was visible 28 days post implantation; Bioluminescent signal could be detected in the animal long before it could be measured with a caliper.
By tagging a tumor with the gene for luciferase, the same gene that allows a firefly to glow, it can produce light that can be monitored in vivo using imaging systems.
In vivo imaging provides researchers with the ability to monitor tumor growth and behaviors in living animals. Visualizing and tracking tumor progression helps researchers understand how therapeutic treatments impact disease.
As a disease progresses and reacts to specific compounds, researchers can measure tumor growth and evaluate the efficacy of compounds in real time. This ability to track disease progression offers a better understanding of how potential drugs will react and impact humans.
This week, Caliper Life Sciences introduced a range of ultra bright light producing tumor cell lines created specifically for in vivo imaging applications.
Dubbed Bioware Ultra, these cell lines are 10 to 100 times brighter than cell lines created using traditional methods, allowing researchers to detect cancer cells (even a single cancer cell) in an animal via non-invasive in vivo imaging. The company believes Bioware Ultra cell lines far exceed the capabilities of previously available technologies.
Mark Roskey, VP of reagents and applied biology at Caliper, commented, “Previously, oncology researchers needed to tag nearly 500 cells before a sufficient signal could be monitored using optical imaging. Bioware Ultra cell lines are the only line of cells available that offers the research community the level of sensitivity to detect a single cell.”
Seven Bioware Ultra cell lines are now available, covering a range of cancer models, including breast, colorectal, lung, prostate and lymphoma.
E. Kevin Hrusovsky, President & CEO of Caliper, discusses the technology in an interview we posted back in December, 2007.