
CryoLife Signs Definitive Agreement to Acquire Cardiogenesis
CryoLife, an implantable biological medical device and cardiovascular tissue processing company, agreed to acquire surgical product maker, Cardiogenesis Corp. for about $22 million.

CryoLife, an implantable biological medical device and cardiovascular tissue processing company, agreed to acquire surgical product maker, Cardiogenesis Corp. for about $22 million.
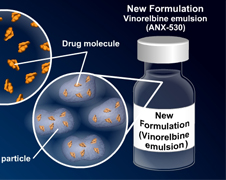
ADVENTRX Pharmaceuticals, Inc. (NYSE Amex: ANX), with its leading innovations in anti-cancer drug development has just announced that they have been successful in sending their ANX-530 (Exelbine) New Drug Application (NDA) to the U.S. Food and Drug Administration (FDA) with a Prescription Drug User Fee Act (PDUFA) date of September 1, 2011.

This year at OneMedForum San Francisco, OneMedPlace offered 17 workshop sessions on a variety of subjects helping investors grow their businesses.

In the halls of government agencies throughout Washington, DC, plans are being made to implement reform measures that will reshape the nation’s healthcare policies—and the health of millions of Americans.

CFO as Enterprise Risk Management Leader: How financial executives add value to this critical process Enterprise risk management (ERM) has the ability to help medical […]

Blue Shield of California, a plan that covers more than three million people, has approved payment for mild to moderate psoriasis treatment using the PhotoMedex […]
Copyright © 2025 | WordPress Theme by MH Themes