
OneMedRadio: Vijay Aggarwal Discusses Diagnostics Trends in 2013 (Part I)
As we approach mid-year, many questions raised in the past year surrounding the state of healthcare company growth development have yet to be answered. The […]

As we approach mid-year, many questions raised in the past year surrounding the state of healthcare company growth development have yet to be answered. The […]

Amedisys, Inc. (AMED) reported 4Q and year-end earnings Tuesday, March 12, and the key highlight was the company’s 2013 EPS guidance range of $0.60-$0.70, substantially below the Street’s $0.77 estimate. Analysts EPS estimates were lowered when the company reported its third quarter 2012 earnings miss (2013 EPS forecasts back then called for $0.93 a share), hence, the business continues to deteriorate and the earnings trough has not yet been reached.

On March 12, 2013, Acadia Pharmaceuticals (ACAD) reported financial results for the fourth quarter and full year 2012. Total revenues in the quarter were $0.4 million, essentially in-line with our estimate of $0.3 million. Revenues consisted of collaborative payments from Allergan, Inc. (AGN) and other collaboration partners. For the full year 2012, total revenues were $4.9 million.
Metastasized breast cancer now has a new therapy on the market, though it remains to be seen whether noticeably dangerous side effects may prevent widespread use. Trastuzumab emtansine (T-DM1) or Kadcyla, produced by Immunogen [Nasdaq: IMGN], is a treatment that targets the HER2 protein.

The Healthcare ecosystem in the United States is changing dramatically due to significant regulations and legislation. Though Obamacare gets the most attention for its impact on patient care and healthcare coverage, legislation such as the Meaningful Use Regulations passed in 2009, followed by the passage of the Affordable Care Act in 2010, and the most recent Sequestration may have a profound impact on the opportunities for developing medical devices and medical device investors.

GlySure Limited, developer of in-hospital continuous blood glucose monitoring systems, today announced that it has achieved ISO 13485 certification, the global standard for the requirements of a quality management system for the design, manufacture and distribution of medical devices. By establishing a quality management system that meets international regulatory and customer requirements, GlySure has set the foundation for its European clinical trials.

WHAT IF there were a way to give cancer a ‘fever’ to enhance the results of chemotherapy and radiation? There is, says Actium BioSystems…

The BIO CEO Investor Conference remains one of the most important events for companies, investors and industry executives to explore partnerships, raise capital, network or simply gain some useful, fresh insight from industry experts. Monday’s opening Plenary Session, Word on the Street–Buy Side View for 2013, was informative and featured some of the industry’s most respected names.
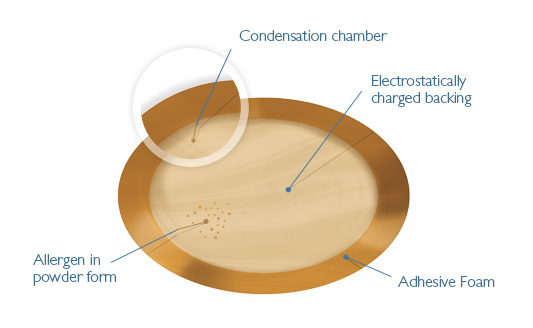
ALLERGY is a burgeoning disease, and food allergies represent the segment where life can be directly threatened. In the U.S., more than three million people are allergic to peanut. Indeed, peanut allergy causes about 100 to 150 deaths each year in the U.S.—but no treatment is currently available. Until now, avoidance of the culprit food has been the primary solution.

Aftab Jamil of BDO spoke with OneMedTV during the 6th Annual OneMedForum in January to discuss the state of life sciences investment, and how companies can formulate new strategies to attract a sustainable class of investors. At the core of these new strategies are the initiatives being implemented to increase access to non-venture capital in the earlier rounds, which may incubate a company long enough to grow a pipeline, attract tenable/diverse revenue opportunity, and eventually reach market.
Copyright © 2024 | WordPress Theme by MH Themes