 It’s been over 18 months since PLC Systems enrolled the first patients in its RenalGuard clinical trial. It now appears the company’s efforts may have been for naught. PLC has deferred the trial pending further funding.
It’s been over 18 months since PLC Systems enrolled the first patients in its RenalGuard clinical trial. It now appears the company’s efforts may have been for naught. PLC has deferred the trial pending further funding.
RenalGuard received CE Mark approval in late 2007; commercialization efforts in Europe are ongoing. The therapy is targeted to patients with diminished renal function who may be at risk for Contrast-Induced Nephropathy (CIN), most commonly defined as renal failure occurring within 48 hours of exposure to radiographic contrast media.
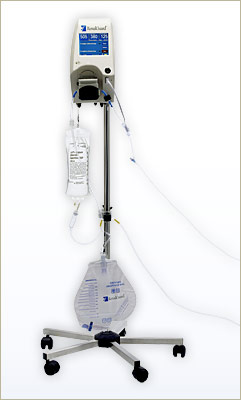
RenalGuard is a real-time fluid replacement device intended for interventional cardiology and radiology patients undergoing imaging procedures using contrast media. Based on existing data, PLC believes initiating and maintaining high urine output will allow the body to rapidly eliminate contrast media, reducing its toxic effects.
Approximately seven million patients worldwide undergo interventional therapeutic and diagnostic procedures each year. CIN is a growing concern due to the increasing number of older patients, diabetics and patients with pre-existing renal failure. They are at increased risk for CIN when they require interventional procedures that use radiographic contrast media. CIN is the third-most common cause of in-hospital acute renal failure.
An investigator-sponsored clinical trial is now underway at the University of Milan in Italy. Mark Tauscher, PLC President and CEO, hopes that results from this study will validate the company’s technology and support fundraising efforts in the U.S.