The human body has a complex mechanism that causes blood to clot if a wound occurs – a desirable response under normal circumstances. But under certain clinical conditions, known as thrombotic disorders, this same mechanism can cause an unwanted clot or “thrombus” and is potentially life threatening.
Prothrombin Time (PT) is the most common way to express the clotting time of blood. The effectiveness of oral anticoagulants (substances that prevent the clotting of blood) can vary over time – variation in diet, alcohol consumption, illness and the use of other drugs can all affect PT. Oral anticoagulant dosages are adjusted according to PT test results; regular monitoring keeps the patient within the desired therapeutic range.
One of the most popular oral anticoagulants is warfarin, a blood thinner used by a large population of patients for a variety of medical conditions. Warfarin use needs to be managed closely in order to minimize serious complications from continual use, including blood clots, stroke and hemorrhage.
Three million people in the U.S. and seven million people worldwide take warfarin daily. For the past several years, use of the drug has grown 10% YOY, driven by an aging population.
Currently, 80 million PT tests are performed worldwide annually. If all patients taking warfarin were tested weekly, the market would be 364 million tests annually.
HemoSense offers the INRatio monitor (the company was acquired by Inverness in November 2007, as part of a stock offering-fueled buying spree). This diagnostic point-of-care system reports PT in a number of formats, including the International Normalized Ratio (INR). The INRatio system reduces the hassle of oral anticoagulation management by obtaining results using fresh capillary whole blood from a fingerstick.
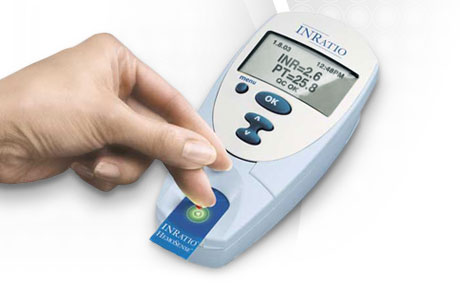
Roche and Thoratec make competing diagnostic systems, but HemoSense claims its version is easier to use. The INRatio monitor offers a one-step process with a simple interface, on-board quality controls and results in only two minutes. Test strips can be stored at room temperature for up to one year.
In 2002, Medicare established reimbursement for point-of-care testing in mechanical heart valve (MHV) patients. The total number of PT/INR tests increased more than 30% between 2000 and 2003, largely as a result of this decision. While there was 11% growth in the laboratory testing market, the point-of-care and patient self-test markets increased by190%.
And last week, a much anticipated announcement came down from the Centers for Medicare & Medicaid Services (CMS) that Medicare coverage for at-home blood testing of PT/INR will be expanded to patients who take anticoagulants daily.
In addition to MHV patients, the new decision expands coverage of home testing to those patients who take warfarin for chronic atrial fibrillation or venous thromboembolism.
Jack Ansell, Chairman of Medicine at Lenox Hill Hospital in New York City commented, “This new CMS decision removes a substantial barrier to the wider use of patient self-testing for INR that we are hopeful will happen in the near future.”