 DBV Technologies, whose shares are traded on segment C of Euronext Paris (Ticker: DBV), believes that its VIASKIN® platform is a game-changer, notably for children and adults who suffer from potentially lethal food allergies. DBV claims that its VIASKIN® technology offers a novel and breakthrough treatment, thanks to a fully proprietary and original method called EPIT (Epicutaneous Immunotherapy) characterized by its safety, thereby opening the door to the treatment of the more severe allergies
DBV Technologies, whose shares are traded on segment C of Euronext Paris (Ticker: DBV), believes that its VIASKIN® platform is a game-changer, notably for children and adults who suffer from potentially lethal food allergies. DBV claims that its VIASKIN® technology offers a novel and breakthrough treatment, thanks to a fully proprietary and original method called EPIT (Epicutaneous Immunotherapy) characterized by its safety, thereby opening the door to the treatment of the more severe allergies
“We believe VIASKIN® will stimulate the interest of the Pharma industry to build an entirely new franchise—a whole new paradigm in the treatment of allergy, even the most severe food allergies, bringing to patients a non-invasive and safe approach to specific immunotherapy,” said Dr. Pierre-Henri Benhamou, co-founder and CEO of DBV Technologies. “Our goal is to make food allergy-specific immunotherapy into a simple pharmaceutical treatment by using a patient’s own skin to solicit the desired immune system reaction while avoiding the risk of life-threatening anaphylactic reactions.”
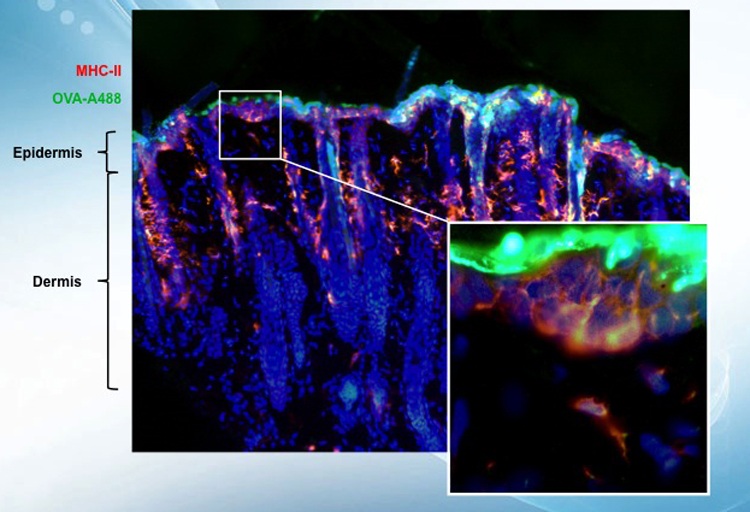
 The safe immunotherapy of patients suffering from dangerous food allergies has not been possible in clinical practice previously, due to the high risk of anaphylactic reactions. The breakthrough and patented design of VIASKIN® presents an allergen onto intact skin via a skin patch while dramatically reducing the risk of the allergen’s free passage into the bloodstream. VIASKIN® thus safely triggers the desired immune reaction via specific immune cells so the body can gradually become desensitized to the allergen—while avoiding the risk of a life-threatening anaphylactic reaction.
The safe immunotherapy of patients suffering from dangerous food allergies has not been possible in clinical practice previously, due to the high risk of anaphylactic reactions. The breakthrough and patented design of VIASKIN® presents an allergen onto intact skin via a skin patch while dramatically reducing the risk of the allergen’s free passage into the bloodstream. VIASKIN® thus safely triggers the desired immune reaction via specific immune cells so the body can gradually become desensitized to the allergen—while avoiding the risk of a life-threatening anaphylactic reaction.
The company’s initial commercialization effort is VIASKIN® Peanut, which is undergoing FDA-Fast-Tracked clinical development. DBV launched in July 2012 the largest study ever in peanut allergy, a worldwide phase IIb clinical trial, called ‘VIPES,’ the first double-blind, placebo-controlled and largest trial ever in desensitization of peanut-allergic children and adults. VIPES (VIaskin® Peanut’s Efficacy and Safety) is a 12-month, multicenter and multinational study conducted in Europe and in North America on 220 randomized patients through approximately 20 investigators. This study is expected to read out in the first half of 2014. This trial is conducted at six U.S. sites: Boston Children’s Hospital, Children’s Hospital Philadelphia, Children’s Memorial Chicago, Massachusetts General Hospital, Stanford University and UT Southwestern University
 “Allergy is a growing disease, and food allergies represent the segment where life can be directly threatened—but no treatment is available,” said David Schilansky, CFO of DBV Technologies. “Until now, avoidance of the culprit food has been the primary acceptable solution. Treatment of food allergies is a significant worldwide, unmet medical need. Indeed, there are 12 million food-allergic people in the U.S. alone, and incidence of peanut allergy has doubled over the past 5 years in children. Since there are no treatments for food allergies, many children and their families live with the constant fear of ingesting a life-threatening food.”
“Allergy is a growing disease, and food allergies represent the segment where life can be directly threatened—but no treatment is available,” said David Schilansky, CFO of DBV Technologies. “Until now, avoidance of the culprit food has been the primary acceptable solution. Treatment of food allergies is a significant worldwide, unmet medical need. Indeed, there are 12 million food-allergic people in the U.S. alone, and incidence of peanut allergy has doubled over the past 5 years in children. Since there are no treatments for food allergies, many children and their families live with the constant fear of ingesting a life-threatening food.”
The goal of ‘desensitization’ is to increase the amount of allergen that the patient can eat or breath without any symptom. Ultimately, the patient could become ‘tolerant’ to the allergen and live normally. Conventional immunotherapy in the form of drops, pills or injections — used for airborne allergens such as pollens and venom such as bee stings — consists of exposing a patient to a controlled amount of allergen; but these ‘conventional’ treatments are too dangerous for desensitizing food-allergic patients because their mechanism of delivery requires entering the bloodstream. A novel technology combining safety and efficacy is desperately needed by food allergists and patients.
“Food allergies can cause death,” concluded Dr. Benhamou. “It was frustrating to me as a medical doctor and pediatrician that the safe desensitization of food-allergic children and adults had not been possible in clinical practice. I was very concerned about the absence of treatment for children suffering from severe food allergy. I was especially obsessed by the consequences for their health and social life as well as their parents’ difficulties for managing the daily risk of systemic reaction and their incredible demand for a safe treatment.”
The VIASKIN® technology platform, as well as its applications, is protected by fourteen patent families encompassing more than 500 patents worldwide. VIASKIN® is a highly versatile technology, able to target the most dangerous allergies such as Peanut, the most vulnerable patients such as young children, and other applications involving the immune system, such as vaccination.
Allergies today affect between 25% and 40% of the adult population and more than 50% of children in developed countries Food allergies are also very prevalent, with 8% of children and 6% of adults estimated to suffer from a food allergy. Specific Immunotherapy is acknowledged by the World Health Organization as the only disease-modifying treatment for allergy. The market of food allergy represents more than $14 billion in developed countries and no pharmaceutical treatments are currently available due to the risks of anaphylactic shock.
“Hence, very large patient populations are awaiting Viaskin, the first pharmaceutical treatment in food and pediatric allergies,” said Mr. Schilansky.