 Competition among makers of electronic medical implants is intense, and the pressure to differentiate products has led to decreased size and new power-hungry features. As a result, most of the 550,000 pacemakers and Implantable Cardiac Defibrillators (ICDs) placed each year inevitably fail due to limited battery life. The majority of patients who receive these implants will outlive their devices and require replacements. Replacement surgeries are not only costly – they also have complication rates three times higher than those of the initial implantation procedure. Endurance Rhythm’s proprietary technology fills the need for longer-lasting medical implants, as well as helping to reduce the size of these devices.
Competition among makers of electronic medical implants is intense, and the pressure to differentiate products has led to decreased size and new power-hungry features. As a result, most of the 550,000 pacemakers and Implantable Cardiac Defibrillators (ICDs) placed each year inevitably fail due to limited battery life. The majority of patients who receive these implants will outlive their devices and require replacements. Replacement surgeries are not only costly – they also have complication rates three times higher than those of the initial implantation procedure. Endurance Rhythm’s proprietary technology fills the need for longer-lasting medical implants, as well as helping to reduce the size of these devices.
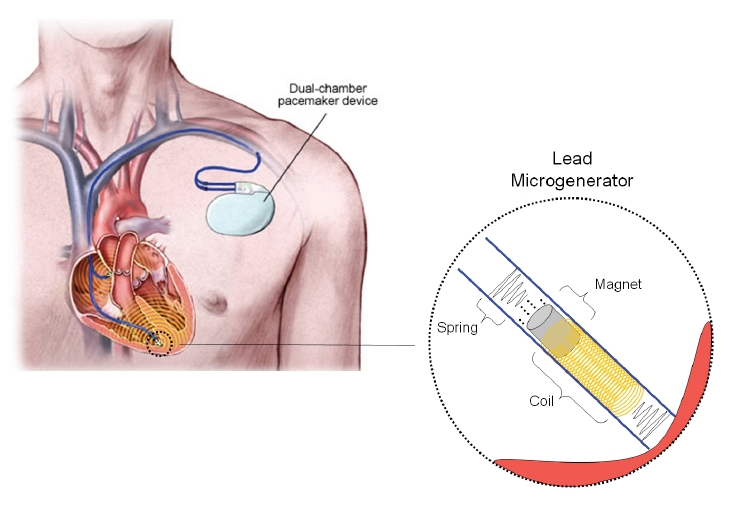
Endurance Rhythm has designed and patented a micro-generator that harnesses kinetic energy from the heart to power electronic implants. Since the heart beats 60-100 times each minute, it provides a regular source of mechanical energy. Pacemakers and ICDs have two major components: a control device that sits underneath the skin near the collarbone, and a multi-wire lead connecting the device to the heart wall.
The Endurance Rhythm micro-generator will be placed within the lead tip. As the heart muscle contracts and moves the lead tip, the generator converts this motion into electricity, providing adjuvant power for the device’s primary battery (see figure above).
The device will not require any change to the existing implantation procedure. In a proof of concept demonstration, a non-optimized prototype exceeded performance expectations, and confirmed that the Endurance Rhythm technology should increase pacemaker lifetime at least two-fold.
The design is simple, fills an unmet need in large and growing markets ($10 billion/yr, where 1% market share translates to $100M in revenues), and benefits patients, physicians, payers, and device manufacturers. Large pacemaker/ICE manufacturers have already expressed significant interest in the technology. Following proof of concept, Endurance Rhythm will be well positioned for an early acquisition, or to pursue multiple longer-term partnering opportunities in multiple markets, including pacemakers, ICDs, and neuro- and GI pacer devices.
The company has developed an early prototype. Within 18 months, Endurance plans to develop an optimized proof of principle design and begin animal testing. The company is currently seeking $1.5M in funding to refine, test, and optimize an advanced at-scale model, engage partners, expand IP coverage, and optimize the business scenarios.