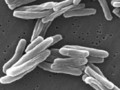
 On Thursday, the U.S. Food and Drug Administration unveiled a new initiative to accelerate the development of cocktail drugs to treat tuberculosis (TB). A number of pharmaceutical companies will collaborate with FDA scientists to test new TB combination therapies and identify promising candidates. The program, called Critical Path to TB Drug Regimens (CPTR), could significantly cut down the time it takes for new tuberculosis treatments to come to market.
On Thursday, the U.S. Food and Drug Administration unveiled a new initiative to accelerate the development of cocktail drugs to treat tuberculosis (TB). A number of pharmaceutical companies will collaborate with FDA scientists to test new TB combination therapies and identify promising candidates. The program, called Critical Path to TB Drug Regimens (CPTR), could significantly cut down the time it takes for new tuberculosis treatments to come to market.
CPTR was developed by the Global Alliance for TB Drug Development, the Critical Path Institute, and the Bill & Melinda Gates Foundation. The alliance is similar to initiatives in the 1990s that helped bring AIDS therapies to the public. Companies involved in the initiative include Johnson & Johnson, Sanofi-Aventis, Pfizer, Novartis, and Sequella. It usually takes about 24 years for a new TB therapy to gain regulatory approval. The new initiative could potentially reduce that time to six years. The current treatment standard for TB is over 50 years old, and must be taken for six months.
Tuberculosis is one of the most widespread global killers. The World Health Organization announced this week that approximately 150,000 people worldwide died of drug-resistant TB in 2008. The WHO estimates that one-third of the world’s population is infected with the bacilli that cause TB, although most people do not experience symptoms. One in ten people will become sick with active TB. Companies working to diagnose TB include Chembio Diagnostics, which develops rapid tests for the early detection of infectious diseases such as TB. Intercell is working on a novel TB vaccine. Immtech International is developing a new class of oral drugs to treat TB and other bacterial infections.