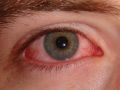
 Adenoviral conjunctivitis, commonly known as “pink eye,” is one of the most contagious and widespread eye diseases. It can be several uncomfortable days before the redness, crusting, and irritation associated with pink eye begin to abate. There is a significant clinical need for a treatment that provides faster relief of symptoms.
Adenoviral conjunctivitis, commonly known as “pink eye,” is one of the most contagious and widespread eye diseases. It can be several uncomfortable days before the redness, crusting, and irritation associated with pink eye begin to abate. There is a significant clinical need for a treatment that provides faster relief of symptoms.
Foresight Biotherapeutics, a pharmaceutical company focused on diseases of the eye and ear, has announced the results of a pre-clinical study of their FST-100 solution to treat adenoviral conjunctivitis. In the study, FST-100 performed significantly better than other treatments for adenoviral conjunctivitis, including the current experimental “gold standard” topical cidofovir.
The study, performed at the Louisiana State University Eye Center, was conducted on 20 rabbits who were injected with human adenovirus type 5. The animals were randomized into groups and treated for eight days with one of four topical solutions: FST-100, 0.5% cidofovir, Tobradex ophthalmic suspension, or a placebo. Researchers looked at factors including eye discharge, eyelid inflammation, and excessive tearing. The rabbits treated with FST-100 showed dramatic signs of improvement within 48 hours. They were the only group to reach complete clinical resolution in the study. The other rabbits experienced inflamed corneas and eyelids, along with conjunctival inflammation.
Cidofovir is made by Gilead Sciences; Tobrodex is a product of Alcon. Another form of conjunctivitis is bacterial conjunctivitis, commonly treated with antibiotic eyedrops such as Merck‘s Gentamicin or Alcon’s Vigamox. In May, the FDA approved Bausch and Lomb‘s Besivance for bacterial conjunctivitis.