Kensey Nash announced the first commercial shipment of its OsseoFit Porous Tissue Matrix to Biomet Sports Medicine, the sports medicine subsidiary of Biomet Inc. The event triggers a milestone payment, which had been planned for the current quarter.
In January 2007, Kensey and Biomet signed an exclusive marketing and distribution agreement for OsseoFit. Under the agreement, Kensey will manufacture and Biomet will market and distribute the product.
OsseoFit Porous Tissue Matrix is an implant manufactured using Kensey’s proprietary Porous Tissue Matrix technology, which creates foamed scaffolds suited for tissue engineering applications. OsseoFit utilizes several resorbable biomaterials including a proprietary collagen formulation, synthetic polymers and ceramics.
“Meeting this milestone is an important accomplishment in achieving full commercialization of this novel product and technology, and we are pleased to have completed this as planned,” said Joseph W. Kaufmann, President and CEO of Kensey Nash. The product kit will be used to expand the clinical use of the OsseoFit beyond a select group of sites that have had access to the product to date.
In 2006, Kensey’s Biomaterials Division was responsible for 39% of the company’s total revenues (or $36.7 million, a 65% CAGR since 1999). In addition to OsseoFit, Kensey manufactures the Vistoss Scaffold Foam, on behalf of OrthoVita; the foam is used in bone graft procedures to aid in the healing response. Over the past five years, Kensey Nash and Orthovita have co-developed five families of bone graft biomaterials resulting in 23 products, with more in development.
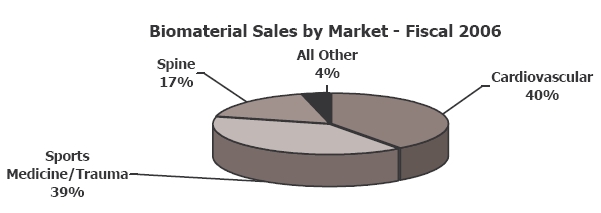
Kensey partners with several companies in the cardiovascular, orthopedics, soft tissue, and spine markets.
Cardiovascular Division
Kensey’s resorbable medical implants include the Angio-Seal Vascular Closure Device, used to close arteries. The device seals femoral artery punctures following catheterization procedures, allowing for shortened hospital stays. Angio-Seal creates a mechanical seal by sandwiching the arteriotomy between a bio-absorbable anchor and collagen sponge, which dissolve within 60 to 90 days. Available since 1996, it was the first resorbable biomaterial component on the market.
Angio-Seal has been marketed and sold by St. Jude Medical since 1999. Kensey receives 6% of all end user sales. The device has a current worldwide market penetration of 45-50%; the vascular puncture market is worth an estimated $1 billion in annual sales. Royalties from Angio-Seal ($19.6 million in fiscal 2006) are a substantial part of the company’s revenues.