 Sunshine Heart, a global company that develops cardiac assist devices, has successfully completed the first two implants of the company’s C-Pulse heart assist system under a 20-person clinical trial approved by the U.S. Food and Drug Administration. The devices were implanted by Dr. Benjamin Sun at the Ohio State University Medical Center in Columbus.
Sunshine Heart, a global company that develops cardiac assist devices, has successfully completed the first two implants of the company’s C-Pulse heart assist system under a 20-person clinical trial approved by the U.S. Food and Drug Administration. The devices were implanted by Dr. Benjamin Sun at the Ohio State University Medical Center in Columbus.
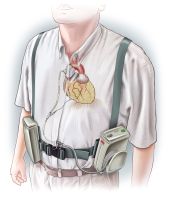
C-Pulse is the only non-blood contacting counterpulsation device designed to treat moderate to severe heart failure. It is implanted via a minimally invasive surgical procedure and can be disconnected at the patient’s convenience. This milestone brings Sunshine Heart one step closer to its goal of addressing the large, unmet clinical need between CRT pacemakers and end stage therapies. Over five million people in the U.S. suffer from heart failure, a progressive condition in which the heart does not pump enough blood. Over 1.4 million of these patients suffer from Class III (or moderate to severe) heart failure, which is classified by the limitation of physical activity. C-Pulse is designed to increase coronary blood flow while reducing the heart’s workload. Sunshine Heart anticipates that the device will significantly improve patients’ quality of life.
Related post: [Device Profile] C-Pulse
Related video: Don Rohrbaugh, CEO of Sunshine Heart