The 1994 bestseller “The Hot Zone” spawned national interest in superbugs, maladies against which the human body is defenseless and for which no effective treatment exist. The book catapulted the Ebola virus into a realm of celebrity most Hollywood A-listers would be envious of.
In the sphere of medical technology, there’s a clear correlation between widespread consumer fear and innovation. Companies, particularly smaller firms, are quick to step in to ease the public’s worries with new product offerings; funding from governmental and private sources goes hand-in-hand with any new superstar superbug.
Take Avian Flu, where public concern peaked sometime in the first quarter of 2005:
- In October, 2005, Quidel released the results of a clinical study that demonstrated its QuickVue Influenza A+B test could also detect avian flu, though the company reiterated, “[the] importance to humans of these viral types is not yet known.”
- In April 2006, Aethlon received a grant from the National Institute to deploy its Hemopurifier as a therapy to clear the Avian Flu virus and viral fragments from a patient’s blood.
- In March 2006, Antigen Express, a Generex subsidiary, announced they were on a fast track with the FDA for a synthetic peptide vaccine to help prepare for a possible avian flu pandemic.
- In December 2006, Nanogen was awarded a $4.5 million contract from the CDC to develop a Point-Of-Care diagnostic assay for Avian Flu, “in support of the US Government’s efforts to strengthen its readiness for a potential influenza pandemic.”
Bird Flu, Ebola and SARS, having experienced their fifteen minutes, have largely fizzled out of the public eye. MRSA, on the other hand, has the benefit of a long, storied past.
From 1992 to 2002, the CDC reports that the percentage of all drug-resistant staph strains, including MRSA, increased from 2% to 63% of the staph infections in the U.S. An August 2006 issue of the New England Journal of Medicine found that MRSA was the most common cause of skin and soft-tissue infections among patients in emergency rooms across the country; the bacteria typically strikes those with weakened immune systems.
Now, the B-Movie star has gotten its big break. MRSA, once confined to hospitals and clinics, is now appearing in locker rooms, on athletes, in prisons, and among military personnel.
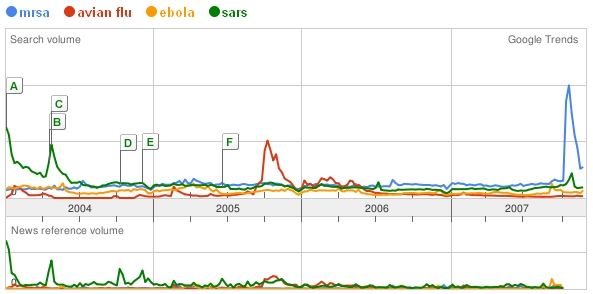
Google Trends shows search volume for these superbugs.
As with Avian Flu, companies are once again capitalizing on the market created by public concern.
Cepheid, gained FDA approval for its MRSA blood, skin and soft tissue diagnostics back in April. This week, the company announced that they’ll be launching GeneXpert in Europe as well. The tests are designed to enable simultaneous rapid detection of two leading causes of hospital and community acquired infections – MRSA and Staphylococcus aureus.
Cantel Medical is also getting into the action through their Crosstex subsidiary. Crosstex waterless hand sanitizers and surface disinfectants are listed by the EPA as effective against a number of bacteria and viruses, including MRSA. The products have held significant market share in healthcare markets for a number of years. The company is now introducing them into other markets, such as schools and businesses to meet the growing demand.
Jay McCann, a Board Member of the Caldwell, NJ school district says, “MRSA and other infections are a big concern to students, parents, and school officials. We need to do all we can to prevent infection, and hand hygiene is the single strongest recommendation we’re hearing.”
With the public’s backing, this star’s got staying power.