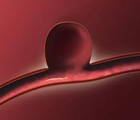
 NeuroVasx’s cPAX Aneurysm Treatment System was approved by the US Food and Drug Administration recently as a humanitarian use device for the treatment of cerebral aneurysms. A cerebral or brain aneurysm is a disorder in which weakness in the wall of a cerebral artery or vein causes a ballooning of the blood vessel.
NeuroVasx’s cPAX Aneurysm Treatment System was approved by the US Food and Drug Administration recently as a humanitarian use device for the treatment of cerebral aneurysms. A cerebral or brain aneurysm is a disorder in which weakness in the wall of a cerebral artery or vein causes a ballooning of the blood vessel.
Christopher Dowd, MD, clinical professor of neuro-interventional radiology at the University of California at San Francisco and medical director for NeuroVasx, notes that the cPAX system will offer physicians an alternative solution to currently available treatments for large, giant, and wide-neck cerebral aneurysms, which are typically the most challenging to treat. Worldwide, cerebral aneurysms occur in an estimated 320,000 individuals annually, and approximately 200,000 cases may be treatable with intracranial surgery using minimally invasive techniques.
The cPAX system uses the same technique as available platinum coil technologies; however, it has a number of advantages over platinum coils. Because of its soft polymeric material, cPAX may provide a more complete filling of the aneurysm and greater long-term stability. Unlike platinum coils, which have a fixed detachment zone, cPAX can detach at any point chosen by the clinician. The continuous filling capability of the product also decreases both the number of devices and the amount of time required for treatment. Further, the polymeric material allows for noninvasive magnetic resonance imaging and computed tomographic scans without metallic artifact, allowing for better patient follow-up assessment.
“The longer-term stability we have seen in the clinical studies using cPAX in larger aneurysms gives me great confidence in the positive impact this product will have on the care of our patients,” states Ricardo Hanel, MD, PhD, associate professor of neurosurgery at the Mayo Clinic in Jacksonville, Florida, and coprincipal investigator in the cPAX clinical trial.
“This FDA approval brings an important cutting-edge technology to a patient population that is currently underserved,” noted Eric B. Timko, president and chief executive officer of NeuroVasx, located in Maple Grove, Minnesota.