OrSense, a privately held Israeli company, has been cleared in Europe to market its first product, a first-in-class, non-invasive glucose monitor.
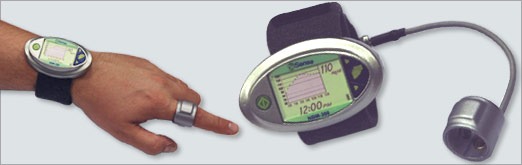
 The NBM-200G allows diabetics to self monitor their glucose levels without having to penetrate the skin. It’s targeted at patients with demanding need for glycemic control, in cases such as brittle, nocturnal and gestational diabetes. In a trial of over 350 diabetics, the NBM-200G exhibited comparable accuracy to invasive monitoring devices. It’s based on OrSense’s patented Occlusion Spectroscopy technology, which correlates blood glucose levels with light-absorption and scattering measurements.
The NBM-200G allows diabetics to self monitor their glucose levels without having to penetrate the skin. It’s targeted at patients with demanding need for glycemic control, in cases such as brittle, nocturnal and gestational diabetes. In a trial of over 350 diabetics, the NBM-200G exhibited comparable accuracy to invasive monitoring devices. It’s based on OrSense’s patented Occlusion Spectroscopy technology, which correlates blood glucose levels with light-absorption and scattering measurements.
According to Globes, an Israeli business daily, the CE Mark certification makes OrSense only the second company (Abbott Laboratories is the other) to bring a minimally invasive glucose monitor to market.
“Many companies have tried unsuccessfully to develop non-invasive devices for monitoring blood glucose levels that would save diabetes patients from having to prick themselves in the fingers several times a day, thereby enabling more frequent testing and a better level of blood sugar monitoring,” Globes reports. “To date, a number of devices have been developed but all have failed efficacy trials on submission for US Food and Drug Administration (FDA) approval. At present, 10 non-invasive and minimally invasive technologies are in the early development stages, the most advanced of which are a minimally invasive technology developed by Abbott Laboratories and OrSense’s device, both of which have now received CE market certification.”
OrSense is already working on a second-generation of the NBM-200G, which will allow diabetics to self monitor glucose levels in a continuous or intermittent mode.
 OrSense investors are led by Israeli VC firms Israel Health Care Ventures and STAR Ventures. Carlo Salvi, Tamar Ventures, Unicycle, Medison, and Lewis Trust Group also are invested in the company.
OrSense investors are led by Israeli VC firms Israel Health Care Ventures and STAR Ventures. Carlo Salvi, Tamar Ventures, Unicycle, Medison, and Lewis Trust Group also are invested in the company.