 Monitoring one’s blood glucose levels is a critical part of managing diabetes. Unfortunately, many patients fail to regularly take this important measurement, citing the pain and inconvenience of jabbing themselves with a needle. Israel-based OrSense is attempting to increase patient compliance with a non-invasive continuous glucose monitor based on optical technology.
Monitoring one’s blood glucose levels is a critical part of managing diabetes. Unfortunately, many patients fail to regularly take this important measurement, citing the pain and inconvenience of jabbing themselves with a needle. Israel-based OrSense is attempting to increase patient compliance with a non-invasive continuous glucose monitor based on optical technology.
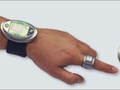
The NBM-200G monitor optically measures blood composition using a patented technology called Occlusion Spectroscopy. A ring-like sensor that slides over the patient’s finger temporarily closes off (occludes) blood flow in the finger, creating new blood dynamics that generate a unique optical signal. Clinicians can analyze the signal to obtain measurements of glucose levels, hemoglobin, oxygen saturation, and other measurements. The NBM-200G monitor has been tested on over 450 patients, with a combined total of over 130,000 glucose readings. The product was able to monitor glucose levels continuously for 24 hours and was found to be highly accurate when compared with gold-standard laboratory measurement systems.
OrSense also offers the NBM-200MP system for the continuous monitoring of hemoglobin and oxygen saturation. Pulse oximetry, the traditional method for measuring oxygen saturation, can provide inaccurate readings in cases of low perfusion, low cardiac output or low blood flow. In a clinical study that measured oxygen saturation, the NBM-200MP system had an overall error level of 2.2 percent, while the pulse oximeters that were tested had an error rate of over 70 percent under weak pulse conditions. The system was also found to be as accurate as invasive point-of-care systems when it came to measuring hemoglobin. Both the NBM-200MP system and the NBM-200G monitor have CE Mark approval in Europe.
OrSense is focused on two major areas: home and hospital care. The company has conducted clinical trials in both critical care and home-like settings. The markets served by OrSense represent over $10 billion in potential annual revenue.