Spinal Restoration, a privately held spine company based in Austin, TX, has closed a $16 million Series B round of equity financing. The money will be used to complete an IDE trial of its Biostat Disc Augmentation System, a biologic treatment for discogenic low back pain.
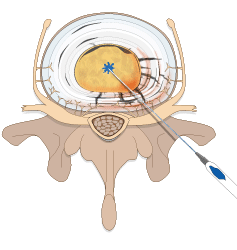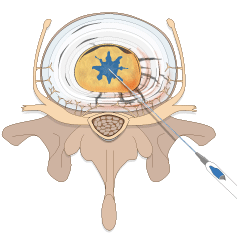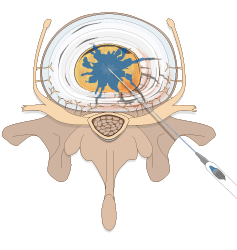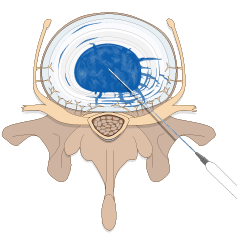 Spinal Restoration’s minimally invasive, outpatient procedure involves application of Biostat BIOLOGX Fibrin Sealant, a human derived biologic compound, into an internally disrupted disc to occlude the annular fissures and restore a level of mechanical integrity to the annulus fibrosus.
Spinal Restoration’s minimally invasive, outpatient procedure involves application of Biostat BIOLOGX Fibrin Sealant, a human derived biologic compound, into an internally disrupted disc to occlude the annular fissures and restore a level of mechanical integrity to the annulus fibrosus.
Occluding the fissures addresses the chemical pain by preventing leakage of the nucleus pulposus into the outer one-third of the annulus fibrosus or onto the spinal nerve root. With the fissures in the annulus occluded, further degradation of the disc may be avoided, stability within the disc is restored, and healing of the disc may be facilitated.
According to the company, more than one million patients suffer from chronic discogenic low back pain each year, yet there is no widely accepted treatment outside of spinal fusion.
“Spinal Restoration has captured a unique solution to an enormous unmet need within the spine health market which offers a very attractive investment opportunity,” said Gary Stevenson, managing partner of MB Venture Partners, in a statement.
MB Venture Partners, along with Santé Health Ventures, joined Series A lead and founding investors Austin Ventures and Path4 Ventures in the B round.
The global orthopedic biomaterials market, valued at $4 billion in 2006, is projected to more than double by 2012, to $8.8 billion, according to a report released last month by Kalorama Information.