A piece from the current issue of The American focuses on three small med tech firms’ efforts to reverse blindness — and the challenges such companies face in garnering funds for their innovative projects.
Neurotech, from Lincoln, RI, is conducting trials for its Encapsulated Cell Technology (ECT), which the company is using to make implants for treating retinal disease. ECT implants consist of cells that are genetically modified to produce a protein that diffuses out of the implant at the target site. It enables controlled, continuous delivery to the retina, bypassing the blood-retina barrier. The implants are designed to help patients suffering from three different eye conditions: degeneration of photoreceptors and/or ganglion cells in the neural retina, vascular proliferation and inflammation.
(See below.) The actual device is 6 mm in length and made from a semi-permeable hollow fiber membrane with a suture loop at one end to anchor the implant to the sclera. The device is surgically placed in the vitreous body, sutured in a manner that allows for retrieval when desired. It’s an out-patient procedure that takes about 25 minutes.
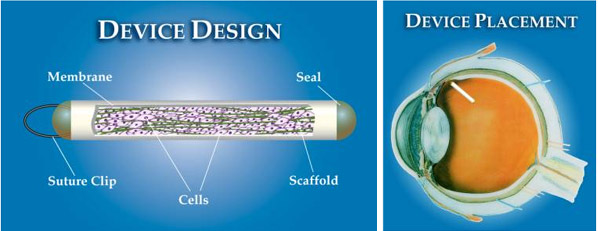
The American reports that Neurotech recently secured $35 million in venture funding to move forward with Phase II trials for the ECT technology.
The other companies mentioned in the article, Optobionics, from Naperville, IL, and Second Sight, of Sylmar, CA, are developing technologies that incorporate electronics.
Optobionics makes the Artificial Silicon Retina (ASR) microchip, designed to stimulate damaged retinal cells, allowing them to send visual signals again to the brain. The company believes the technology has the potential to help patients suffering from retinitis pigmentos, age-related macular degeneration, and possibly other retinal conditions. The 2 mm (diameter) silicon chip that’s implanted under the retina (see below) contains approximately 5,000 microscopic solar cells called “microphotodiodes,” each with its own stimulating electrode. These microphotodiodes are designed to convert the light energy from images into electricalchemical impulses that stimulate the remaining functional cells of the retina.
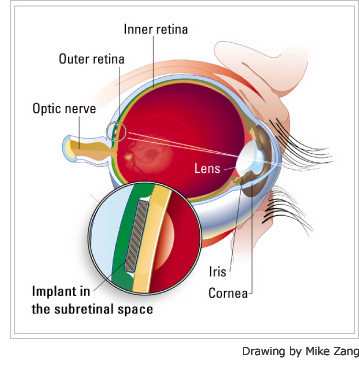
Optobionics has been conducting trials for the ASR technology since June 2000. According to the company’s Web site, so far “no patient has shown signs of implant rejection, infection, inflammation, erosion or retinal detachment related to the implanted microchip.”
Second Sight’s device addresses retinitis pigmentos. It consists of a tiny camera and transmitter mounted in eyeglasses, an implanted receiver, and an electrode-studded array that is secured to the retina with a microtack the width of a human hair. It is powered by a belt-worn microprocessor and battery pack. The camera on the glasses captures an image and sends the information to the video processor, which converts the image to an electronic signal and sends it to the transmitter on the sunglasses. The implanted receiver wirelessly receives this data and sends the signals through a tiny cable to the electrode array, stimulating it to emit electrical pulses. The pulses induce responses in the retina that travel through the optic nerve to the brain, which perceives patterns of light and dark spots corresponding to the electrodes stimulated. Patients learn to interpret the visual patterns into meaningful images.
After detailing the advances being made by these three companies, The American columnist Nick Shulz reflects on the financial challenges commonly faced by small medical technology companies like Neurotech, Optobionics and Second Sight. “At the heart of all these evolving technologies is a long, complex, and expensive discovery process,” says Shultz. “Finding funds for diseases like choroideremia and RP is difficult because any successful treatment will be costly to develop and the market for treatments will be small.”