Staar Surgical has received approval from China’s Food and Drug Administration to market its Visian Toric Implantable Collamer Lens for the treatment of nearsightedness and astigmatism. Chinese regulatory authorities also approved Staar’s Visian ICH, an implantable collamer lens to correct hyperopia or farsightedness.
An implantable contact lens (ICL) is inserted into the eye during a short surgical procedure; a small incision is made and the lens is placed between the iris and the cornea.
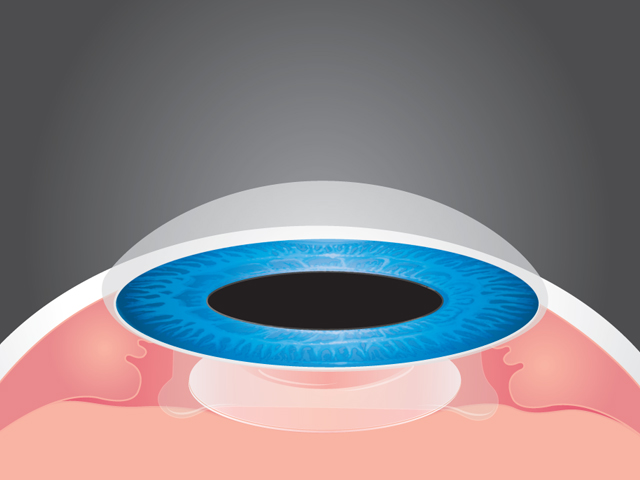
While many patients opt for laser vision correction, some prefer the reversible option provided by implantable lenses. Staar received a CE Mark for its first ICL in 1997. In December 2005, the product was approved by the FDA for the treatment of nearsightedness. The ICL has been implanted in 60,000 eyes to date.
According to Market Scope, a provider of ophthalmic market data, there are over 500 million nearsighted and some 50 million farsighted individuals in China, making it the largest potential refractive market in the world.
In South Korea, a population with a rate of nearsightedness similar to that of China, implant procedures, such as those offered by Staar, are estimated to have reached 7% of the total LASIK market.
Staar has had significant success in the South Korean. Its ICLs are used in approximately 60% of all implant procedures. “Our goal in China over time is to at least replicate the market penetration we are achieving in South Korea,” said Barry G. Caldwell, Staar’s President and CEO.