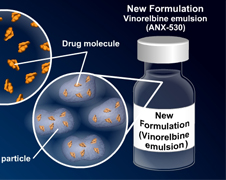
DARA Bio: A Vastly Undervalued Emerging Story
In late March 2013, DARA Bio (DARA) filed its Form 10K annual report highlighting both the business and financial updates from 2012. Below we discuss the key drivers at DARA, and why we believe the company is a vastly undervalued emerging specialty pharmaceutical story.