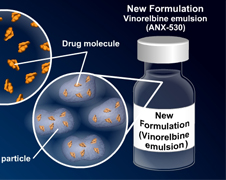
ADVENTRX Pharmaceuticals Takes the Next Step In Approval of ANX-530
ADVENTRX Pharmaceuticals, Inc. (NYSE Amex: ANX), with its leading innovations in anti-cancer drug development has just announced that they have been successful in sending their ANX-530 (Exelbine) New Drug Application (NDA) to the U.S. Food and Drug Administration (FDA) with a Prescription Drug User Fee Act (PDUFA) date of September 1, 2011.
