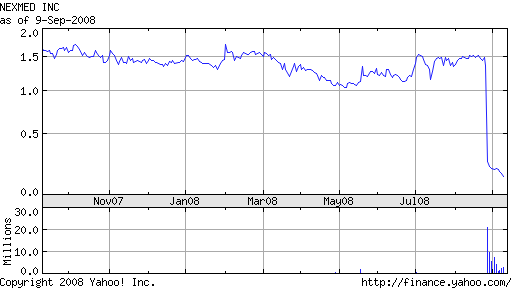
After NexMed announced it would shelve its previously filed NDA for a toe nail fungus (TNF) treatment following disappointing Phase III clinical results, shares of the company slid to their lowest point in 13 years.
In a September 2005 agreement, NexMed had entered into an exclusive, worldwide agreement with Novartis, under which Novartis assumed all clinical development, regulatory, manufacturing and commercialization responsibilities for the drug.
Novartis did not release any information regarding specific results of the Phase III trials, though it did say no significant adverse event was reported. In a conference call, NexMed CEO Vivian Liu said it could take 2 to 3 months before Novartis completed its analysis and released a final report.
Given the onslaught of TNF treatments hitting the market, Novartis’ decision to halt Phase III trials might have had less to do with lackluster results and more to do with an increasingly competitive marketplace.
- In August, PathoLase, Inc. introduced a patented laser technology that kills the pathogens that cause TNF. With $6M of private equity capital recently secured from Nexus Medical Partners, PathoLase is now in discussions with podiatrists in over 20 major metropolitan markets within the U.S.
- In September, HemCon signed an agreement with Institute of Technology Sligo, Ireland, to license a controlled release hydrogen peroxide technology. The technology has broad spectrum antibacterial and antiseptic properties and has particular promise as a treatment for fungal nail infection.
- This week, Nomir Medical Technologies announced positive in vitro and in vivo human data for its Noveon direct optical energy device for the treatment of TNF. Treatment with the Noveon device resulted in 100% photo-inactivation of TNF at safe energy densities and temperatures.
TNF affects an estimated 30 million Americans. The current market for therapeutics is estimated at about $2 billion worldwide.
While the company will not receive anywhere near the $51 million in payments it once sought, NexMed is due to receive an additional $3 million from Novartis, pending a patent milestone. A European comparator study is still ongoing – those results are expected mid 2009.
With low operating costs and cash on hand, I wouldn’t count NexMed out just yet. Given its market capitalization of +/- $10 million this week (the company was worth more $100 million prior to the news), it’s easy to see the share price increase pending some (any?) positive development that reinforces the value of NexMed’s core technology, NexACT.
Disclosure: I took a small position in NexMed this week.