Aethlon Medical has initiated a research collaboration to study the in vitro effectiveness of the Aethlon Hemopurifier to clear HIV from blood.
The studies, being conducted in collaboration with ImQuest Biosciences, will document the rate at which the Hemopurifier captures infectious forms of HIV, including multi-drug resistant strains of the virus. The studies will also identify the capture rate of gp120, a toxin shed from the surface of HIV, which causes apoptosis (programmed cell death) of immune cells. Depletion of immune cells is the hallmark of AIDS.
The Hemopurifier is targeted as an adjunct therapy to extend and enhance the benefit of antiviral drug regimens by inhibiting the proliferation of drug resistant HIV and other immunosuppressive toxins. In addition, Hempurifier will function as a non-drug therapy to maintain viral load suppression during structured treatment interruptions known as “drug holidays”.
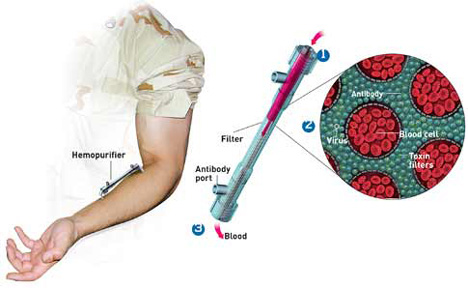
Since June of 2001 Aethlon has focused exclusively on the development of the Hemopurifier. The device, which resembles a modified kidney dialysis cartridge, exploits the pre-existing global infrastructure of dialysis and continuous renal replacement machines already present in hospitals and clinics.
The Hemopurifier rapidly separates and captures infectious viruses and toxins from circulating blood before the occurrence of cell and organ infection. The mechanism of capture occurs through the polysaccharide chains that reside on the surface glycoproteins of envelope viruses. While viral proteins may vary greatly in their arrangement, polysaccharides chains are nearly invariant, solving the problem presented by mutations and drug resistance. Hemopurifier has been shown to inhibit the growth of all tested strains of HIV and can capture of inhibit the growth of other envelope viruses including SIV, FIV, HCV, Measles, Mumps, Influenza, Ebola, Marburg, and Orthopox viruses.
In April 2006, Aethlon completed a human clinical trial to demonstrate the safety of the Hemopurifier at the Apollo Hospital in New Delhi, India. This study showed no adverse treatment affects amongst the patients, all of whom suffered from both end stage renal disease and Hepatitis-C infection. Researchers also observed an approximate 30% capture of circulating HCV during each examined four-hour treatment.